sur ABIVAX (EPA:ABVX)
Abivax Announces Positive Phase 3 Results for Obefazimod in Ulcerative Colitis
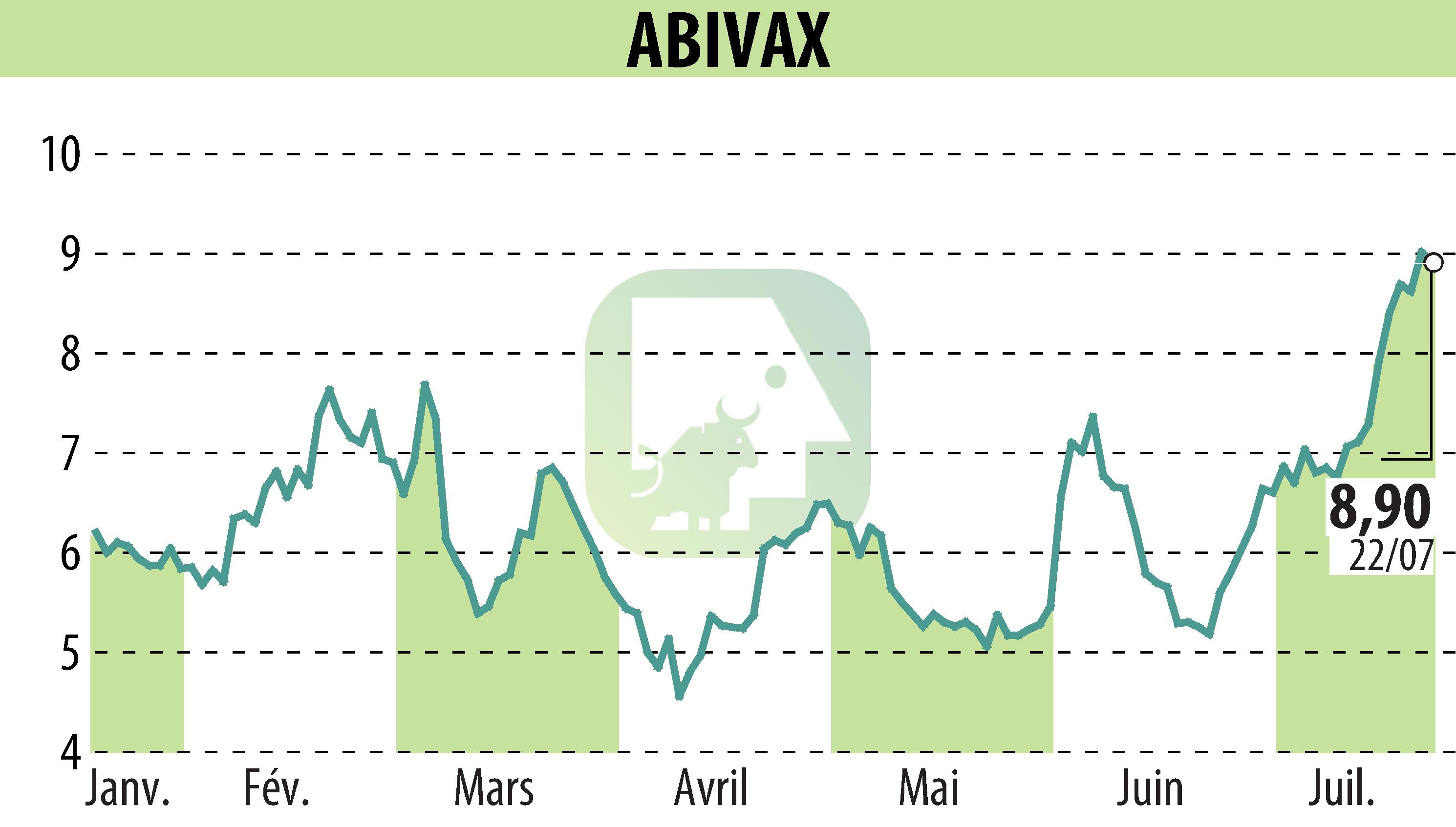
Abivax disclosed positive Phase 3 results from its ABTECT 8-week induction trials for Obefazimod, a miR-124 enhancer, targeting ulcerative colitis. The trials, ABTECT-1 and ABTECT-2, demonstrated that a 50 mg daily dose achieved a pooled placebo-adjusted clinical remission rate of 16.4% by Week 8, with the trials individually showing 19.3% and 13.4%, respectively. Key secondary endpoints were also met.
The trials included a diverse participant base, with nearly half previously unresponsive to advanced therapies. Obefazimod maintained a favorable safety profile. The ongoing ABTECT Maintenance Trial's results are expected by Q2 2026.
Abivax plans to submit a New Drug Application to the FDA in late 2026, contingent on the maintenance trial's success. The company will host a webcast to further discuss these findings.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de ABIVAX
