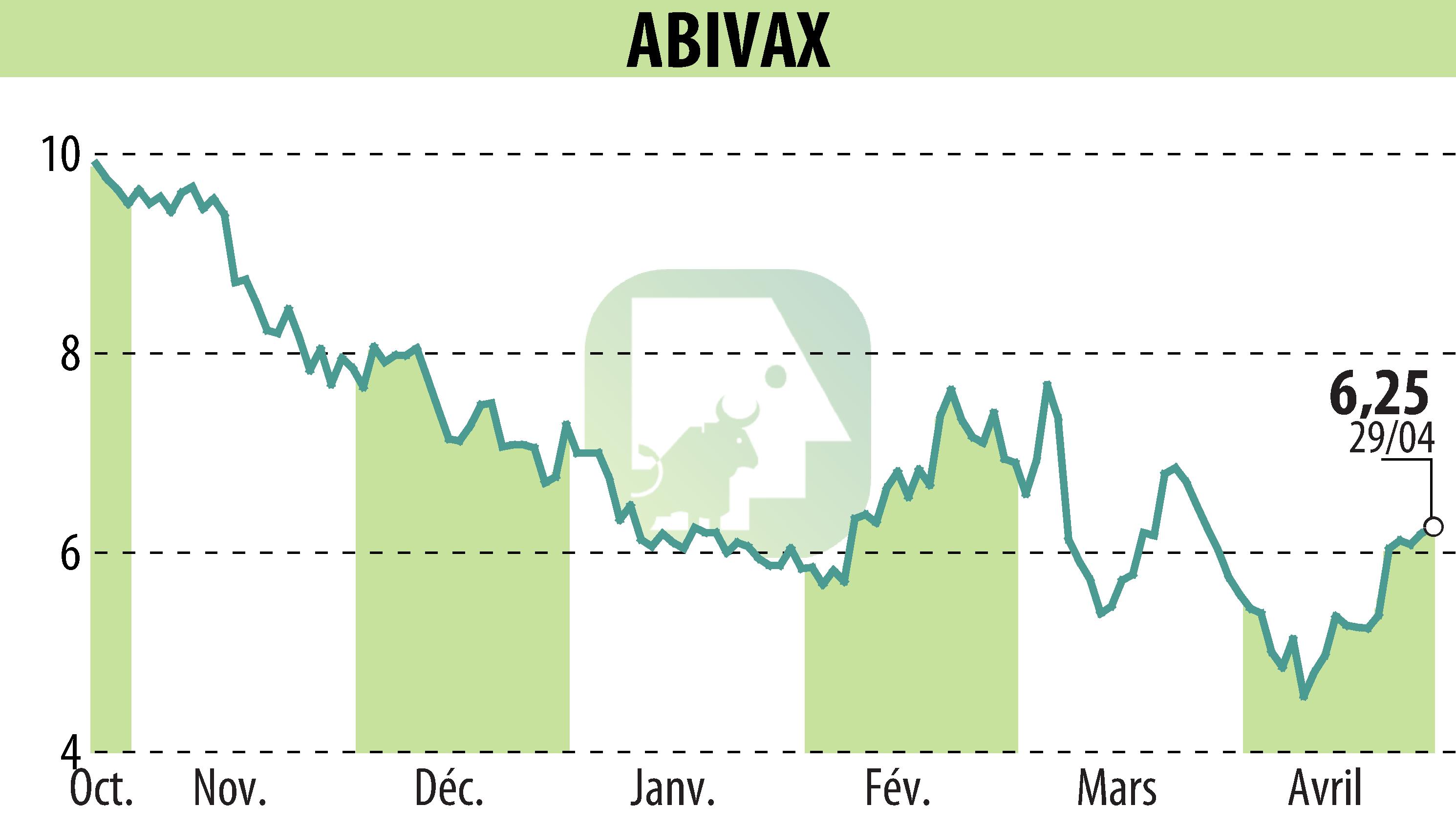
sur ABIVAX (EPA:ABVX)
Abivax Completes Enrollment for Phase 3 Ulcerative Colitis Trials
Abivax has successfully completed enrollment for its Phase 3 ABTECT trials, focusing on obefazimod for moderately to severely active ulcerative colitis. Initially aiming for 1,224 participants, the trial surpassed expectations by enrolling 1,275 patients—an achievement marking a milestone in ulcerative colitis research.
The trials, spanning Studies 105 and 106, anticipate releasing top-line results from the 8-week induction phase in Q3 2025. A comprehensive set of 44-week maintenance data is expected by Q2 2026. Following successful results, Abivax plans to submit a New Drug Application (NDA) in the US during the second half of 2026.
The characteristics of participants align with the Phase 2b UC trial, supporting confidence in obefazimod's efficacy and safety profile. Notably, the cash runway is expected to support this endeavor into Q4 2025, with no new safety signals reported.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de ABIVAX
