sur ABIVAX (EPA:ABVX)
Abivax Reaches Key Enrollment Milestone in Phase 3 ABTECT Trial
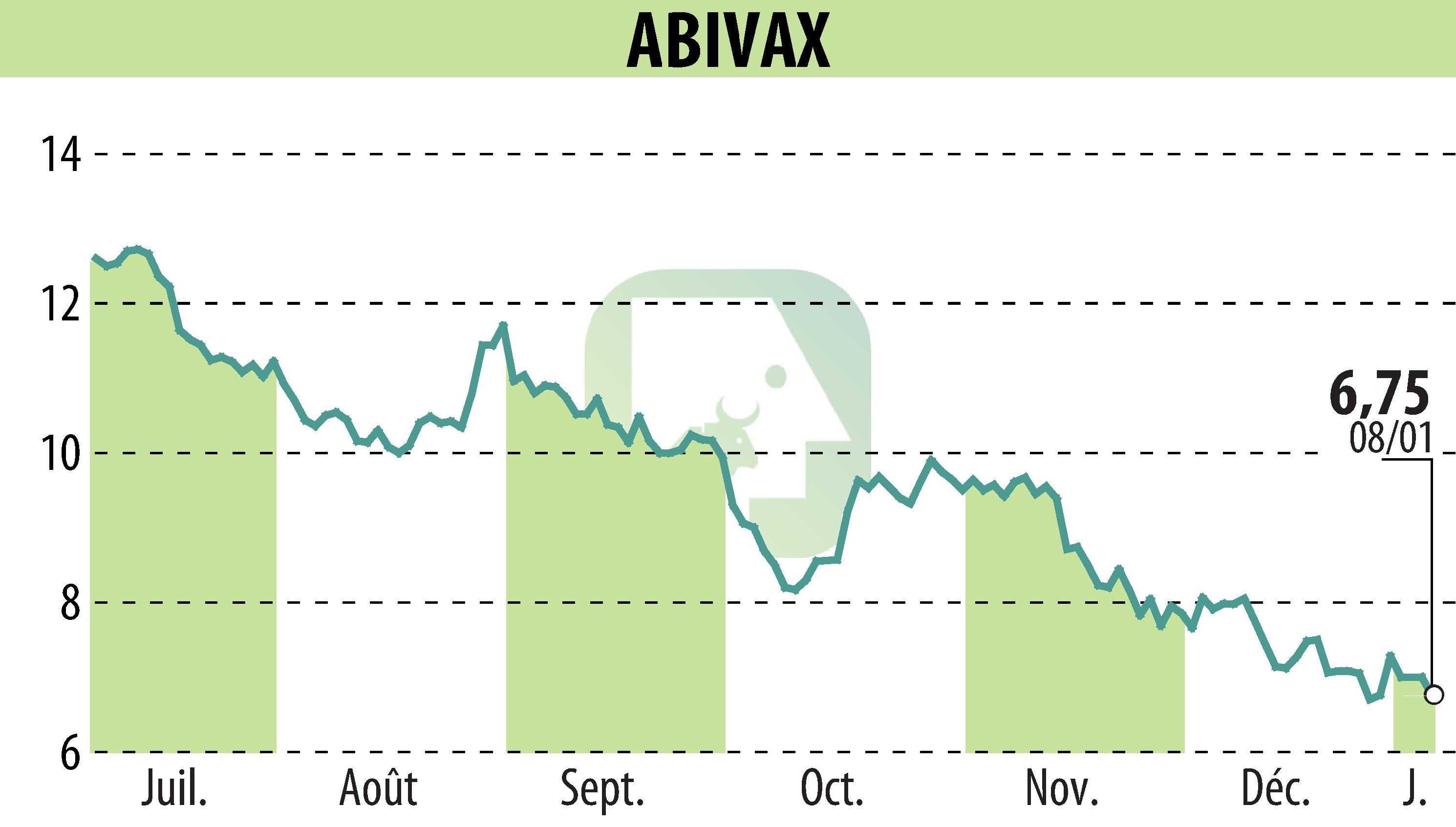
Abivax has announced a significant milestone in its Phase 3 ABTECT clinical trial, designed to evaluate obefazimod for moderately to severely active ulcerative colitis (UC). The trial has enrolled 1,003 of the targeted 1,224 participants, achieving 82% of its enrollment goal. The company expects to complete enrollment by Q2 2025.
Top-line results from the 8-week induction trial are anticipated in Q3 2025, with the 44-week maintenance data projected for Q2 2026. Assuming positive outcomes, a New Drug Application (NDA) submission is planned for the latter half of 2026. Current participant data aligns with the target population from the Phase 2b trial, and no new safety concerns have been observed.
Marc de Garidel, CEO of Abivax, highlighted the swift progress of the trial and its potential to confirm obefazimod as a first-in-class oral treatment for UC, noting the significant unmet need it aims to address. CFO Didier Blondel affirmed the company’s financial stability through Q4 2025 without the need for additional funding.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de ABIVAX
