sur Aspire Biopharma, Inc. (NASDAQ:PWUP)
Aspire Biopharma Receives IRB Approval for Rapid-Acting Aspirin Trial
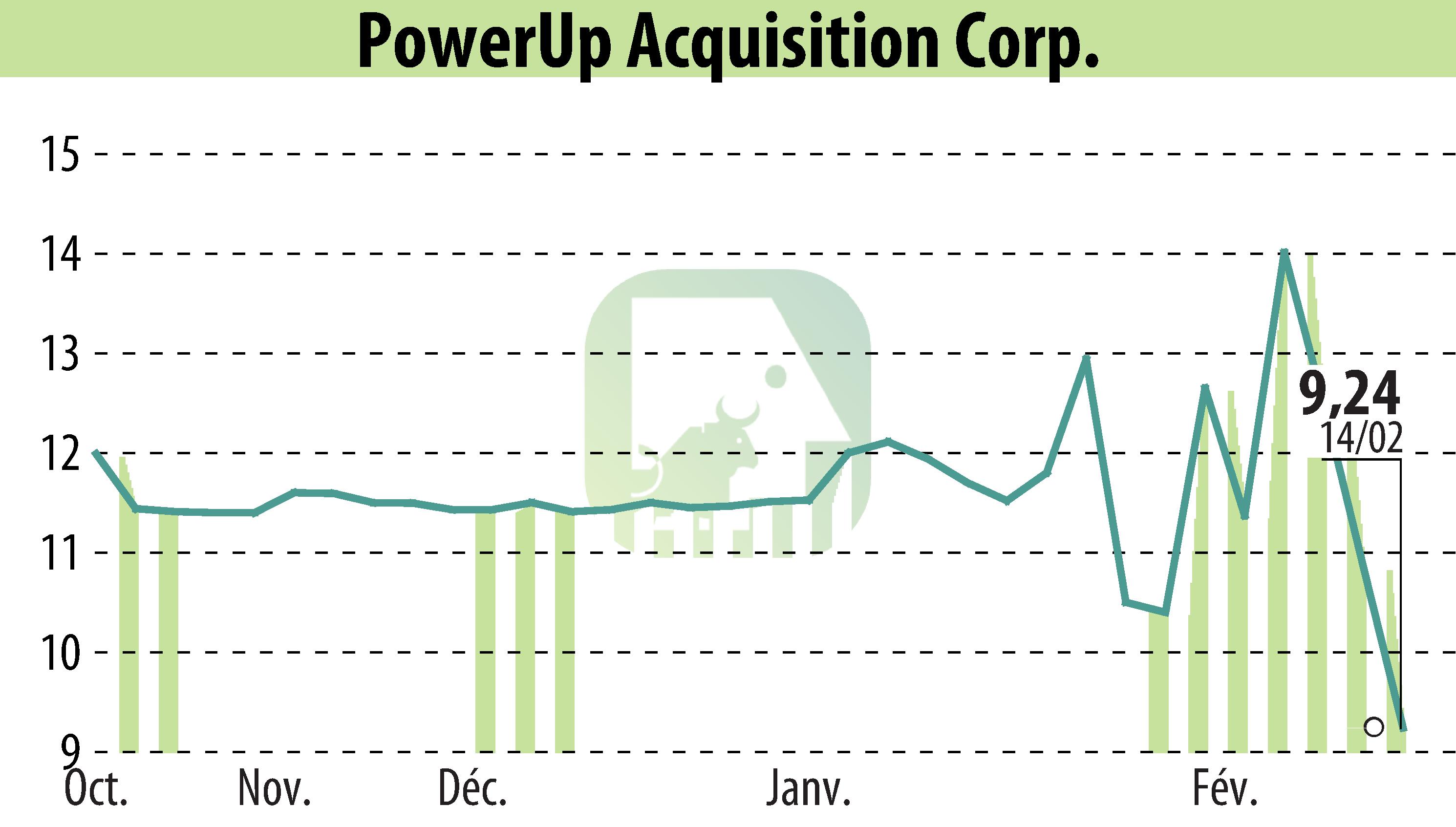
Aspire Biopharma Holdings, Inc. has announced Institutional Review Board (IRB) approval for its upcoming Phase I clinical trial in the U.S. This trial will evaluate safety, pharmacokinetics, and pharmacodynamics of a new oral transmucosal fast-acting high-dose aspirin formulation. Enrollment is set for 6-8 participants with trials beginning imminently.
The trial will compare pharmacokinetic and pharmacodynamic data between volunteers given sublingual aspirin powder and those receiving standard oral aspirin. This study will inform future trials and potential FDA fast-track approval for treating suspected acute myocardial infarction.
The sublingual formulation is designed for rapid absorption, aiming to improve treatment outcomes, especially in emergency cardiac situations. Greater bioavailability may also enhance aspirin's effects while reducing gastric irritation risks.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Aspire Biopharma, Inc.
