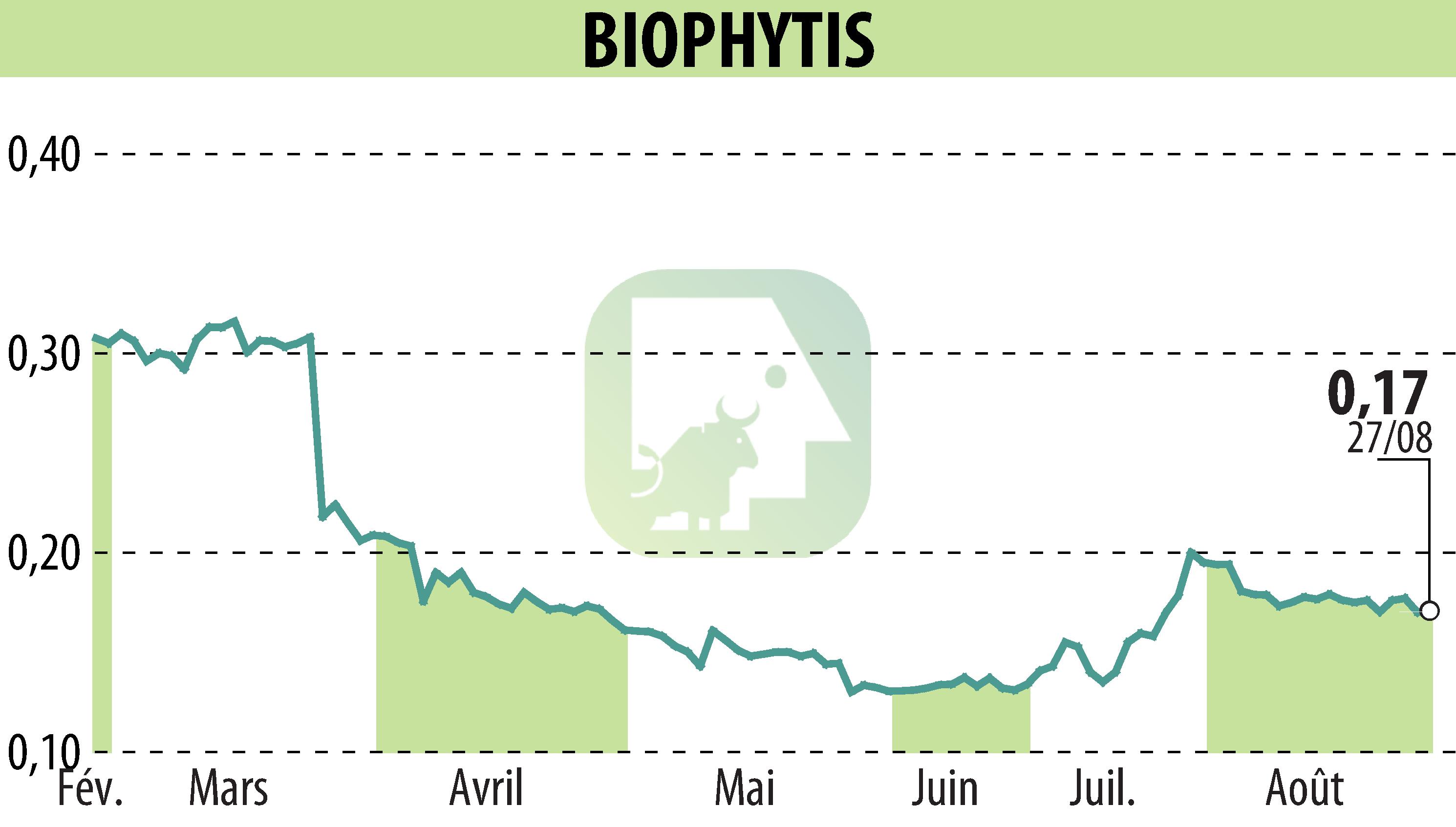
sur Biophytis (EPA:ALBPS)
Biophytis Gains Regulatory Approval for Phase 3 Sarcopenia Trial
Biophytis SA has received the green light from both the European Medicines Agency (EMA) and Belgian authorities to commence a Phase 3 clinical trial for sarcopenia. This approval covers both the scientific validity and ethical considerations of the trial, allowing patient enrollment in Belgium and collaboration with clinics across Europe.
The EMA's Part I review confirmed the study's adherence to rigorous scientific standards, evaluating study protocols, risks versus benefits, and product quality. Concurrently, Belgium's Part II assessment addressed local ethical standards, including consent, data protection, and participant safety, ensuring compliance with national regulations.
CEO Stanislas Veillet noted the approval as a significant step in providing effective treatments for age-related muscle loss. The trial will proceed with the drug candidate BIO101, reflecting Biophytis’ ongoing dedication to combating sarcopenia and other age-related conditions.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Biophytis
