sur EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Receives Not-Approvable Letter from FDA for VenoValve
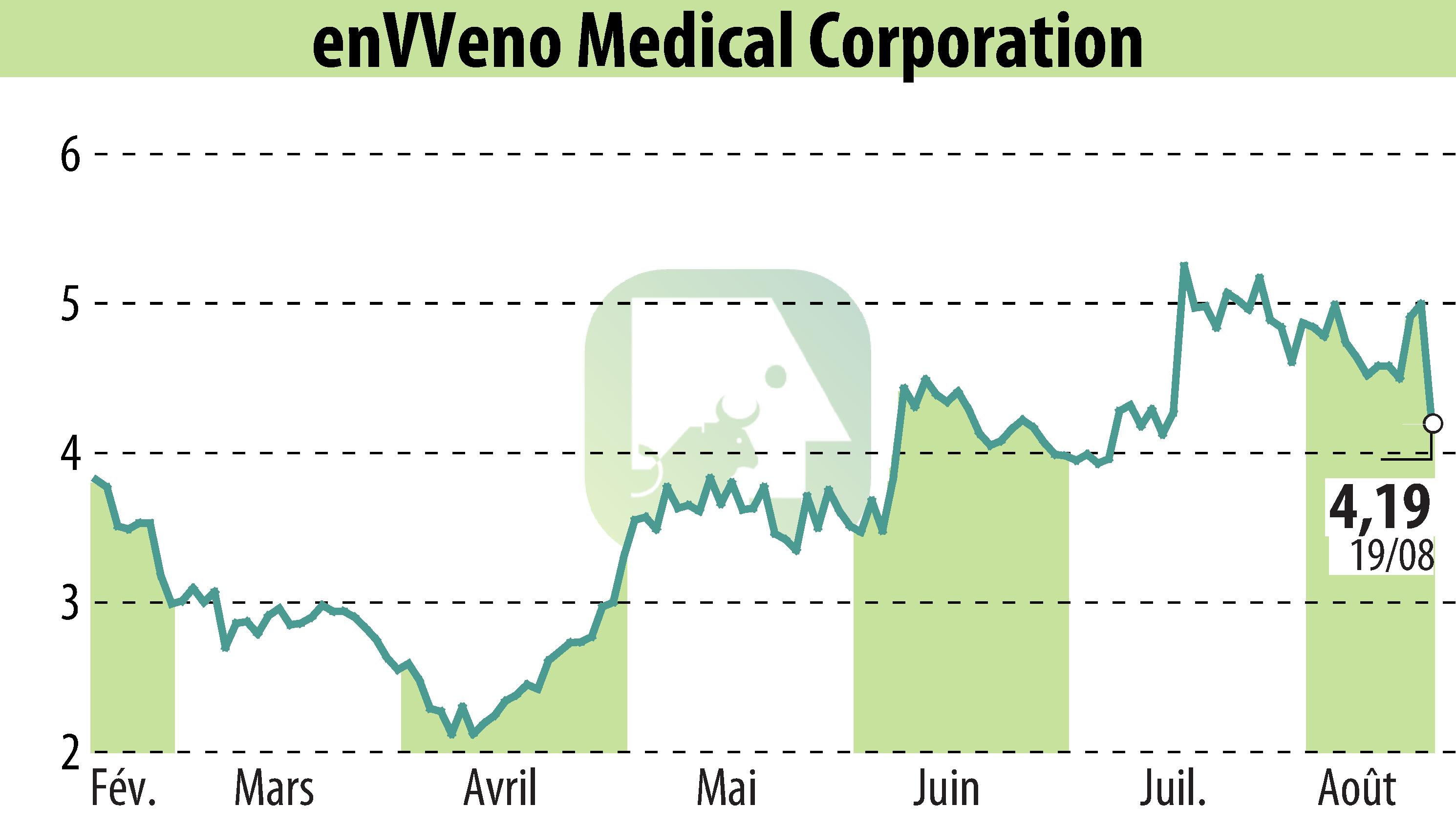
enVVeno Medical Corporation has announced receipt of a not-approvable letter from the FDA for its VenoValve®. This decision follows the FDA's review of the Premarket Approval application, citing concerns over insufficient data to prove the device's benefit-risk profile. Despite favorable clinical improvement data, the absence of specific hemodynamic measurements raised concerns over potential bias in the study results.
Safety issues related to the surgical procedure and subsequent re-hospitalizations also influenced the FDA's conclusion. enVVeno, however, remains committed to addressing these concerns and plans to work with the FDA on demonstrating safety and effectiveness.
The company is considering all options including discussions with the FDA for resubmission or potential appeal. enVVeno aims to apply insights gained from this process to advance enVVe, a non-surgical option currently under development.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de EnVVeno Medical Corporation
