sur EnVVeno Medical Corporation (NASDAQ:NVNO)
EnVVeno Medical Reveals Promising Interim Results from VenoValve Study
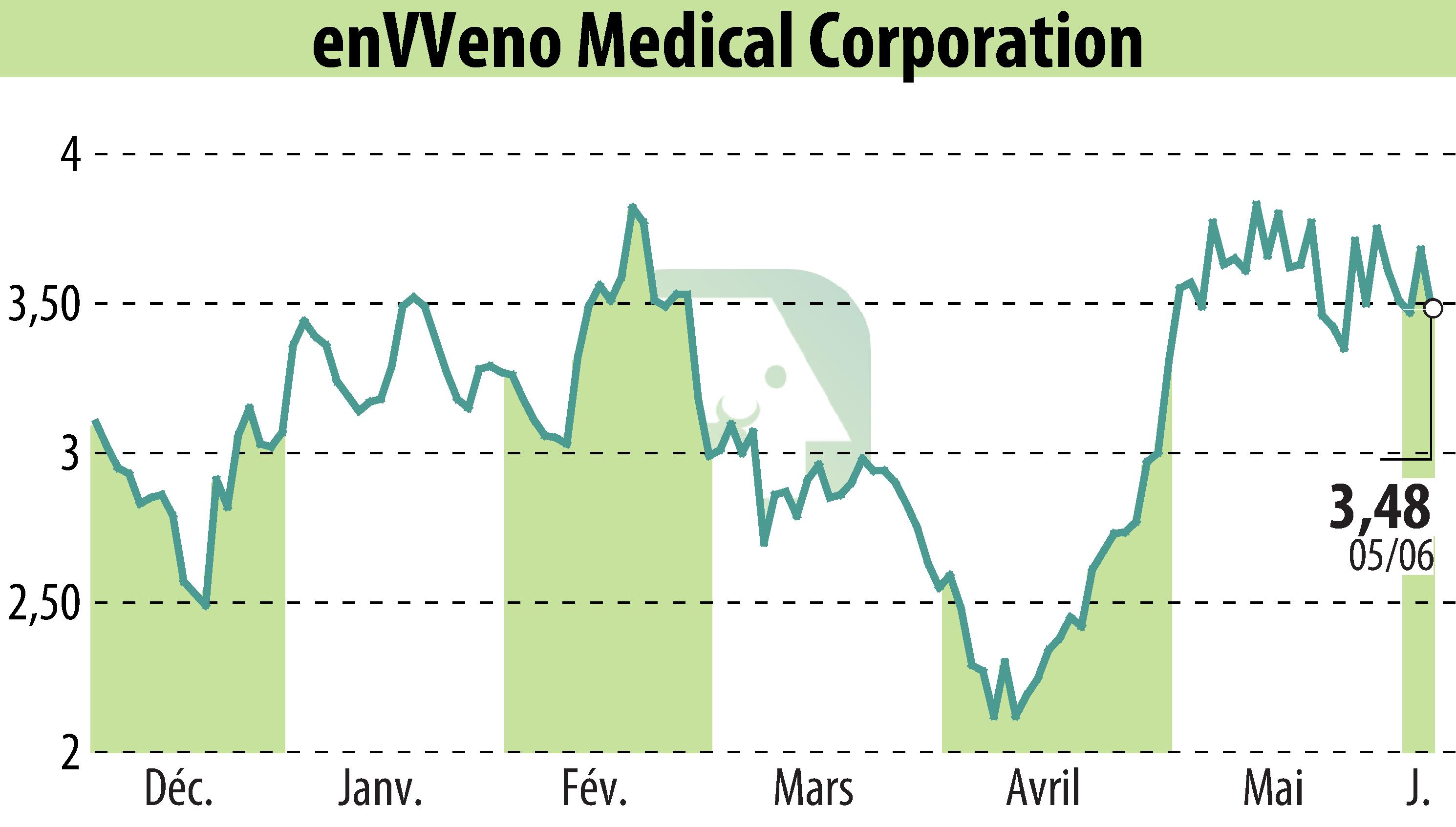
EnVVeno Medical Corporation has announced positive interim two-year data from its VenoValve pivotal trial. Out of 42 participants, 83% maintained a clinically significant improvement in the Revised Venous Clinical Severity Score (rVCSS), with an average enhancement of 9.1 points within the responsive cohort. Participants also reported a median 74% decrease in leg pain, as measured by the Visual Analog Scale. Additional patient-reported outcomes highlighted sustained enhancements across all venous-specific quality-of-life measures.
The data were presented at the Society for Vascular Surgery’s 2025 Vascular Annual Meeting in New Orleans. The company awaits a decision from the FDA on its pre-market authorization application for the VenoValve in the latter half of 2025. The VenoValve is positioned as a potential breakthrough in treating severe chronic venous insufficiency (CVI) caused by malfunctioning deep vein valves.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de EnVVeno Medical Corporation
