sur GENFIT (EPA:GNFT)
GENFIT's Positive Phase 2 Results for Elafibranor in PSC Announced
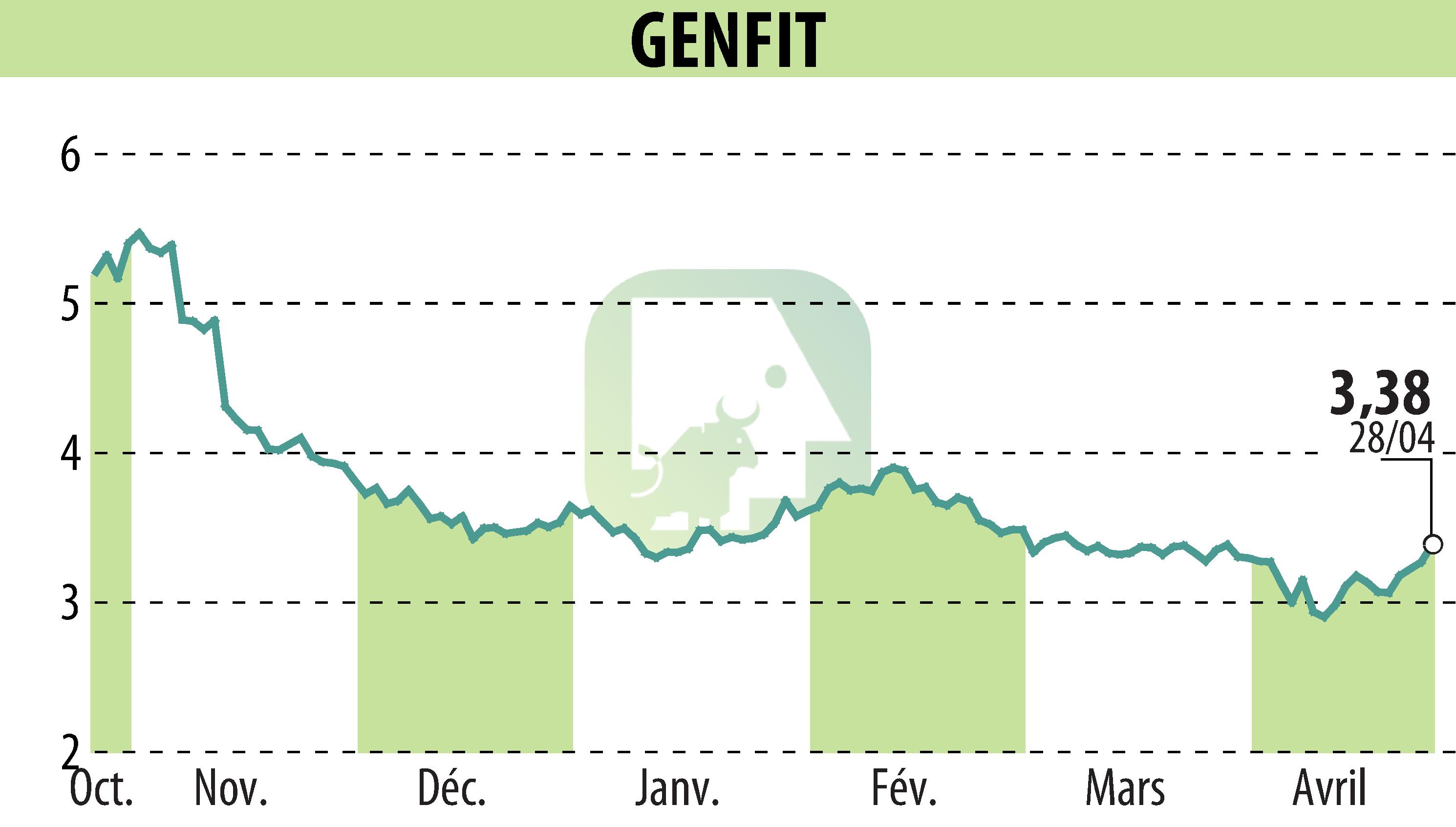
On April 28, 2025, GENFIT revealed that Ipsen would present positive Phase 2 data on elafibranor for Primary Sclerosing Cholangitis (PSC) at the EASL Congress. Elafibranor showed significant efficacy, notably reducing alkaline phosphatase levels in the ELMWOOD trial. Patients taking 80 mg and 120 mg dosages experienced notable improvements as early as week 4 and stabilization of Enhanced Liver Fibrosis at week 12.
Elafibranor, known as Iqirvo®, is already marketed for Primary Biliary Cholangitis (PBC) and is noted for its favorable safety profile. The drug was developed by GENFIT and licensed to Ipsen. GENFIT continues to focus on liver diseases, believing in elafibranor's potential to address significant unmet needs.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de GENFIT
