sur GENSIGHT BIOLOGICS S.A. (EPA:SIGHT)
GenSight Biologics Reports Positive Five-Year Outcomes for LUMEVOQ® Gene Therapy
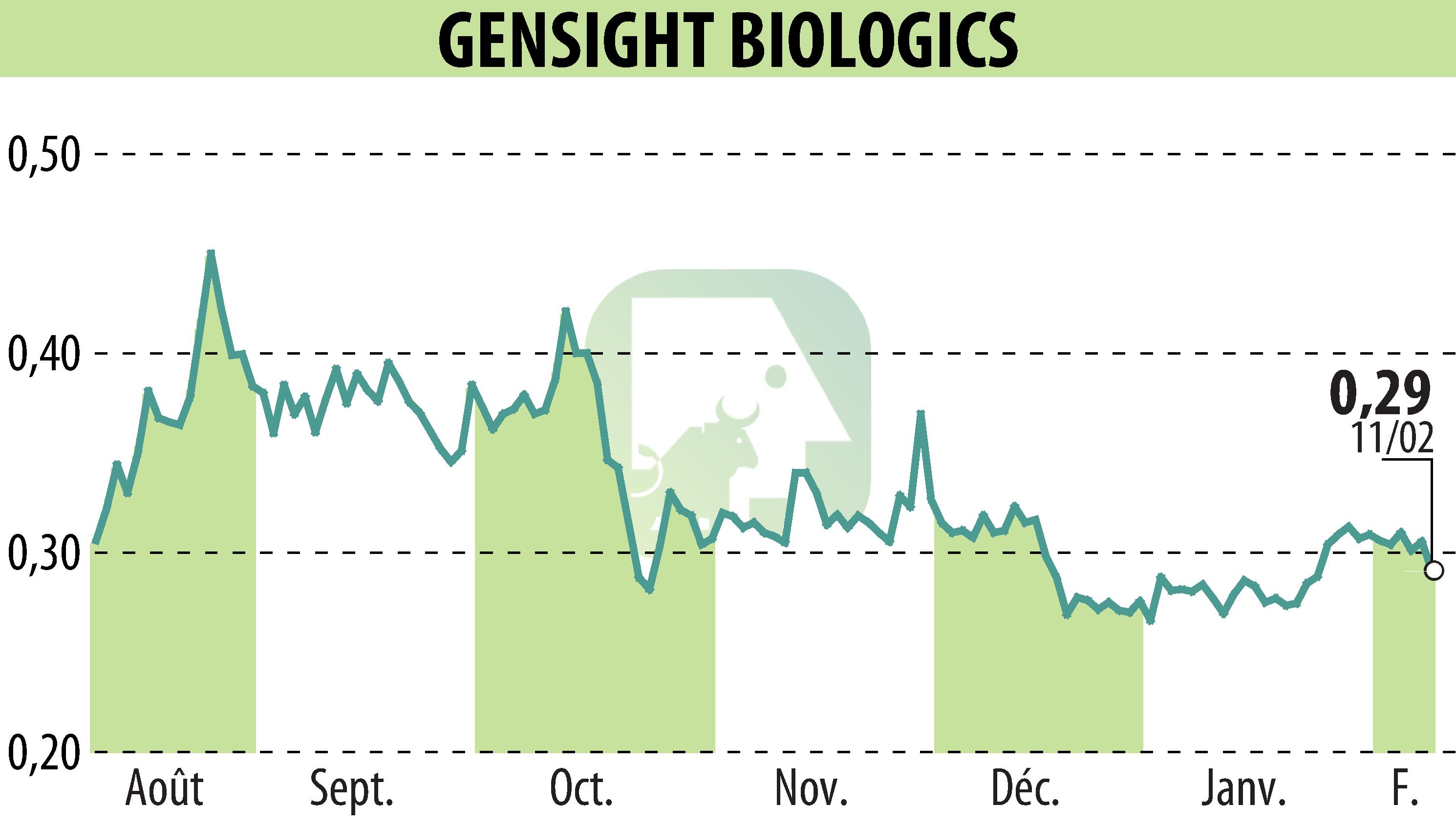
GenSight Biologics announced that its Phase III REFLECT trial has demonstrated sustained efficacy and a favorable safety profile for LUMEVOQ® in treating Leber Hereditary Optic Neuropathy (LHON) over five years post-injection. Visual acuity notably improved, with 75% of bilaterally treated patients experiencing significant recovery versus their nadir. The trial found that bilateral injections offered enhanced benefits compared to unilateral treatment.
Participants receiving bilateral injections reported a clinically meaningful improvement in visual acuity, exceeding the conventional +15-letter threshold. LUMEVOQ®'s safety was reaffirmed, showing no severe adverse events barring mild intraocular inflammation. This reinforces previous observations and highlights the gene therapy's long-term potential in managing retinal neurodegenerative conditions.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de GENSIGHT BIOLOGICS S.A.
