sur Heidelberg Pharma AG (ETR:HPHA)
Heidelberg Pharma Advances HDP-101 in Clinical Trial for Multiple Myeloma
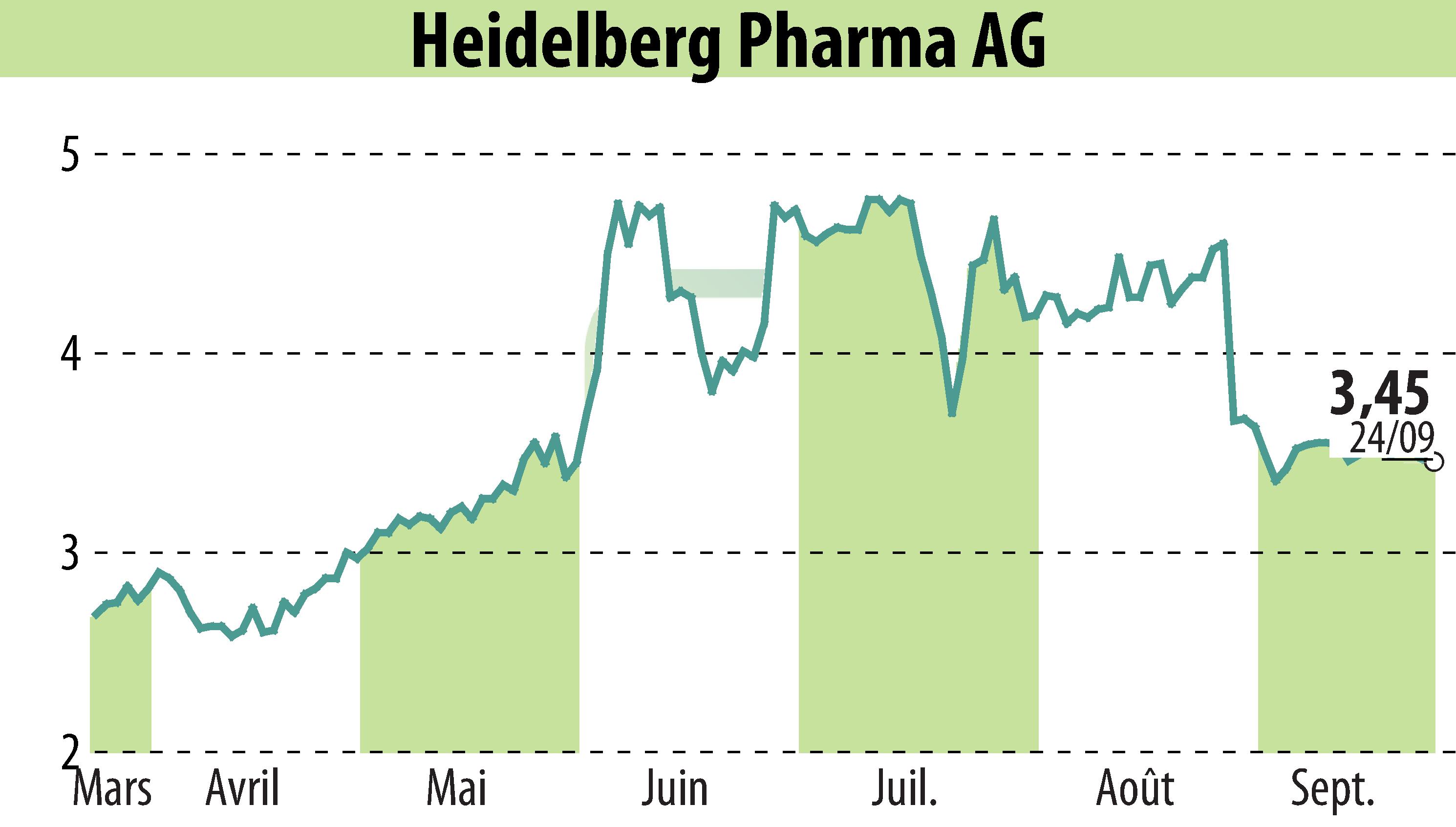
Heidelberg Pharma AG has initiated Cohort 9 in its Phase I/IIa trial, focusing on HDP-101, a lead Antibody Drug Conjugate (ADC) candidate for treating relapsed or refractory multiple myeloma. This progression follows a successful Cohort 8, where no dose-limiting toxicities were reported, and a favorable safety profile was maintained.
An escalated dose of 175 µg/kg is now administered in Cohort 9. Notably, Cohort 8 demonstrated early evidence of clinical activity, with one patient experiencing a very good partial response. This adds to the success of Cohort 5, where a patient achieved complete remission. Such outcomes underline HDP-101's therapeutic potential in heavily pretreated patients.
The study aims to assess HDP-101's safety, tolerability, and efficacy in patients with multiple myeloma or other BCMA-expressing disorders, contributing valuable insights into the treatment of this challenging condition.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Heidelberg Pharma AG
