sur Heidelberg Pharma AG (ETR:HPHA)
Heidelberg Pharma Reveals Promising Data for HDP-101 at ADC Congress 2025
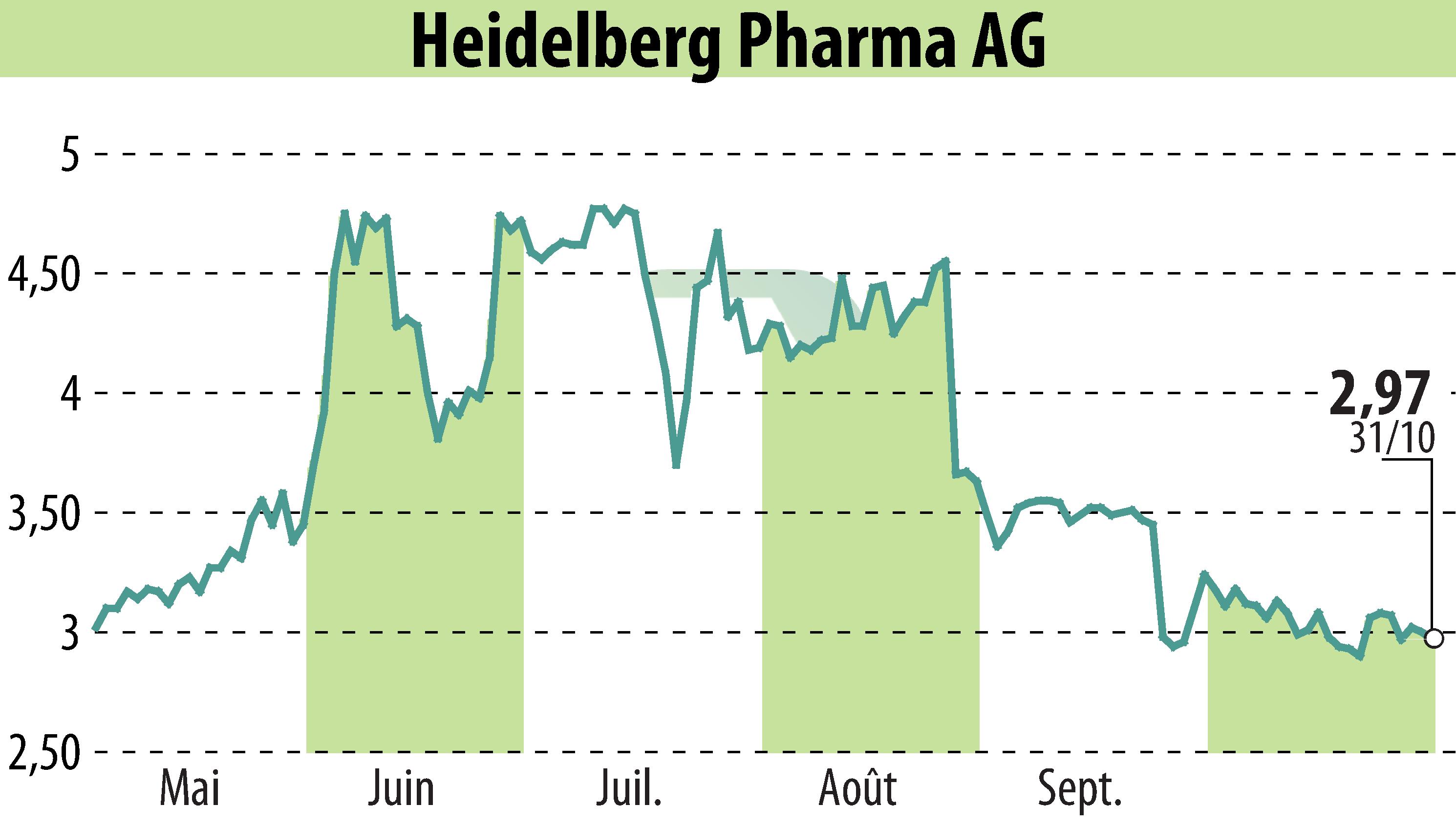
Heidelberg Pharma AG has announced new clinical data for their leading Amanitin-based ADC candidate, HDP-101, at the World ADC Congress 2025 in San Diego. This data from the ongoing Phase I/IIa trial highlights the encouraging anti-tumor activity and safety profile of HDP-101 in treating relapsed or refractory multiple myeloma. The drug targets anti-BCMA, addressing significant needs in bone marrow cancer therapy.
Phase I results are focused on determining optimal dosage levels. CEO Professor Andreas Pahl will update on this at the congress on November 5. A subsequent R&D webinar on November 11, featuring Professor Marc-Steffen Raab, will allow interaction with investors and analysts about HDP-101's potential and future directional strategies.
Heidelberg Pharma's effort is part of a broader focus on developing innovative ADC technologies to treat cancer. The company remains committed to advancing its clinical programs and exploring strategic partnerships for future growth.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Heidelberg Pharma AG
