sur Heidelberg Pharma AG (ETR:HPHA)
Heidelberg Pharma to Unveil Clinical Data on HDP-101 at EHA 2025
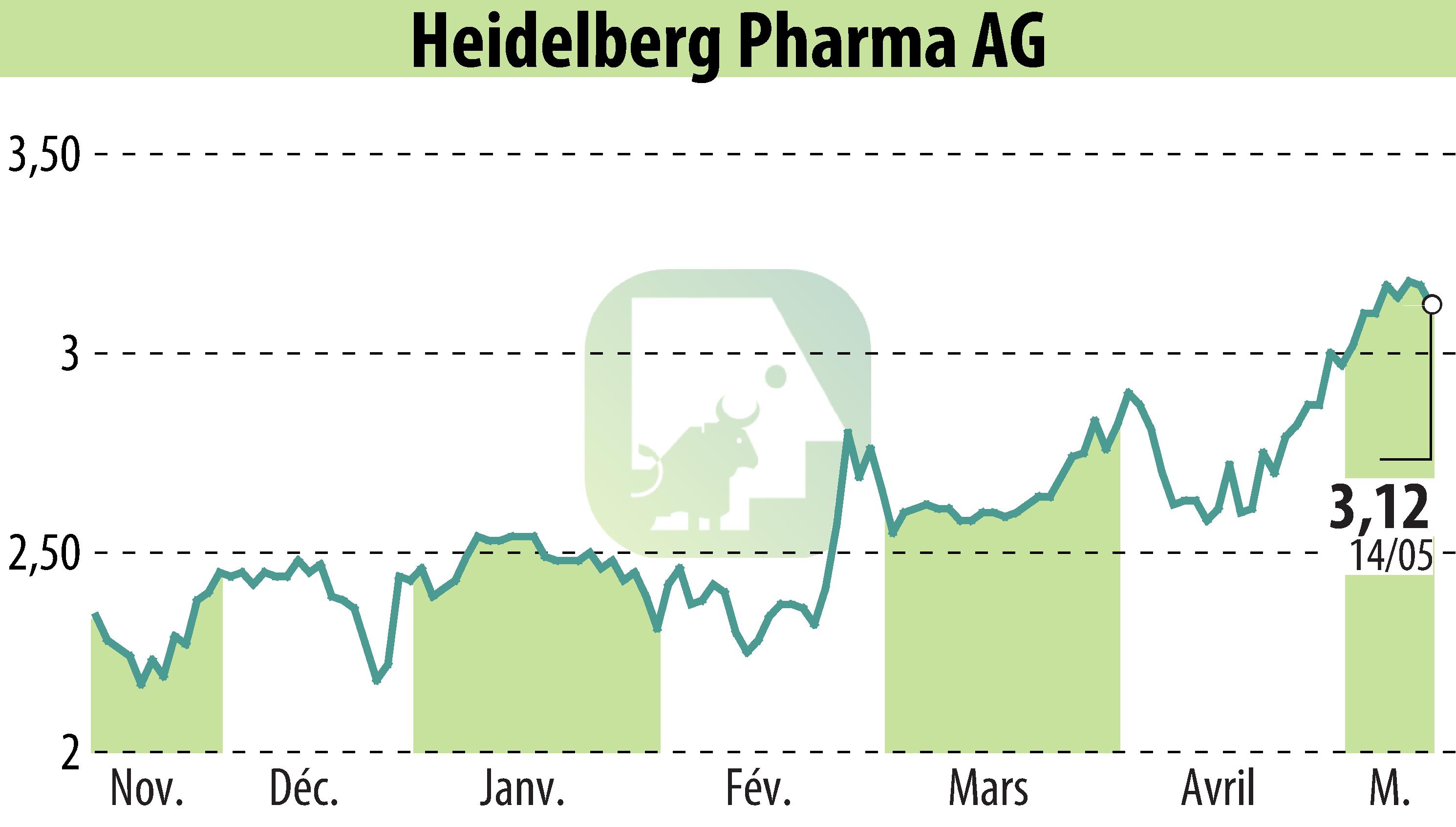
Heidelberg Pharma AG is set to present new clinical data on HDP-101, its lead Amanitin-based Antibody Drug Conjugate (ADC) candidate, at the European Hematology Association Congress in Milan from June 12-15, 2025. This revelation comes as part of the biotech firm's ongoing Phase I/IIa clinical trial for treating relapsed or refractory Multiple Myeloma.
The clinical trial has shown promising results, including a complete response in a female patient who was heavily pretreated. HDP-101 exhibits encouraging biological activity and objective improvements in several patients. The study continues to advance, currently in cohort 8 at a dose of 140 µg/kg.
Following the congress, Heidelberg Pharma will host an R&D webinar on June 17, discussing the ongoing trial with input from Key Opinion Leaders in Multiple Myeloma treatment. This event aims to provide greater insights into HDP-101's potential as a treatment option.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Heidelberg Pharma AG
