sur Heidelberg Pharma AG (ETR:HPHA)
Heidelberg Pharma's HDP-101 Advances in Multiple Myeloma Trial
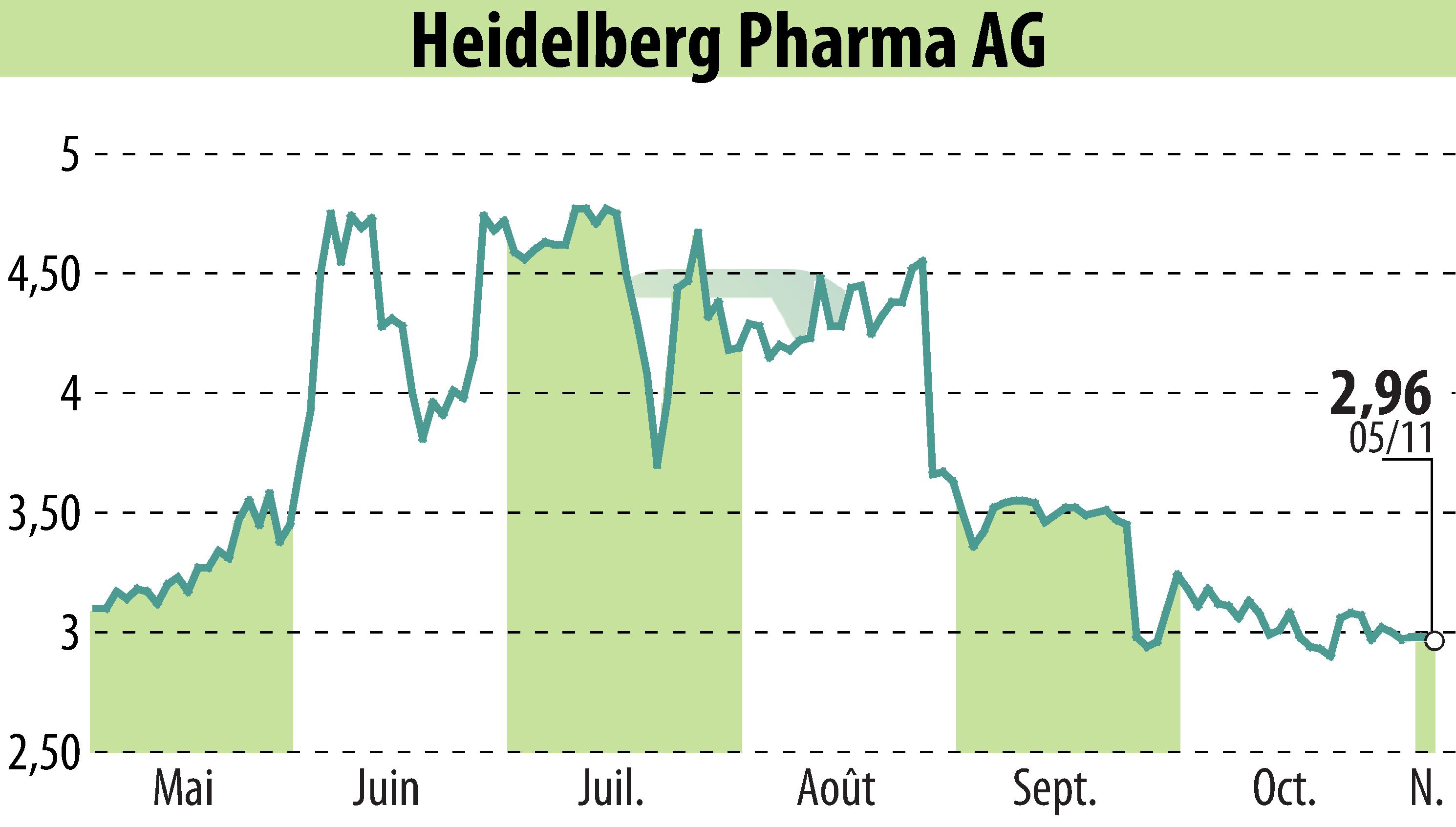
Heidelberg Pharma AG has reported progress from its Phase I/IIa trial of the lead ATAC candidate, HDP-101, for relapsed or refractory multiple myeloma. In Cohort 8, significant clinical activity was observed, including stringent complete remissions in two patients. These results build on previous findings and demonstrate both efficacy and a favorable safety profile for HDP-101, with no dose-limiting toxicities recorded.
Cohort 8 involved seven patients treated at a 140 µg/kg dose level. Four patients exhibited biological activity, including partial and very good partial responses, alongside the notable complete remissions. Dr. András Strassz, Heidelberg Pharma’s Chief Medical Officer, highlighted this as an encouraging validation of their therapeutic approach.
The trial is advancing with Cohort 9 at a higher 175 µg/kg dose as the company continues to evaluate HDP-101's potential. An R&D webinar on 11 November 2025 will provide further insights from the Heidelberg Pharma team and other experts.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Heidelberg Pharma AG
