sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Commences Pivotal Crofelemer Trial for SBS-IF and MVID
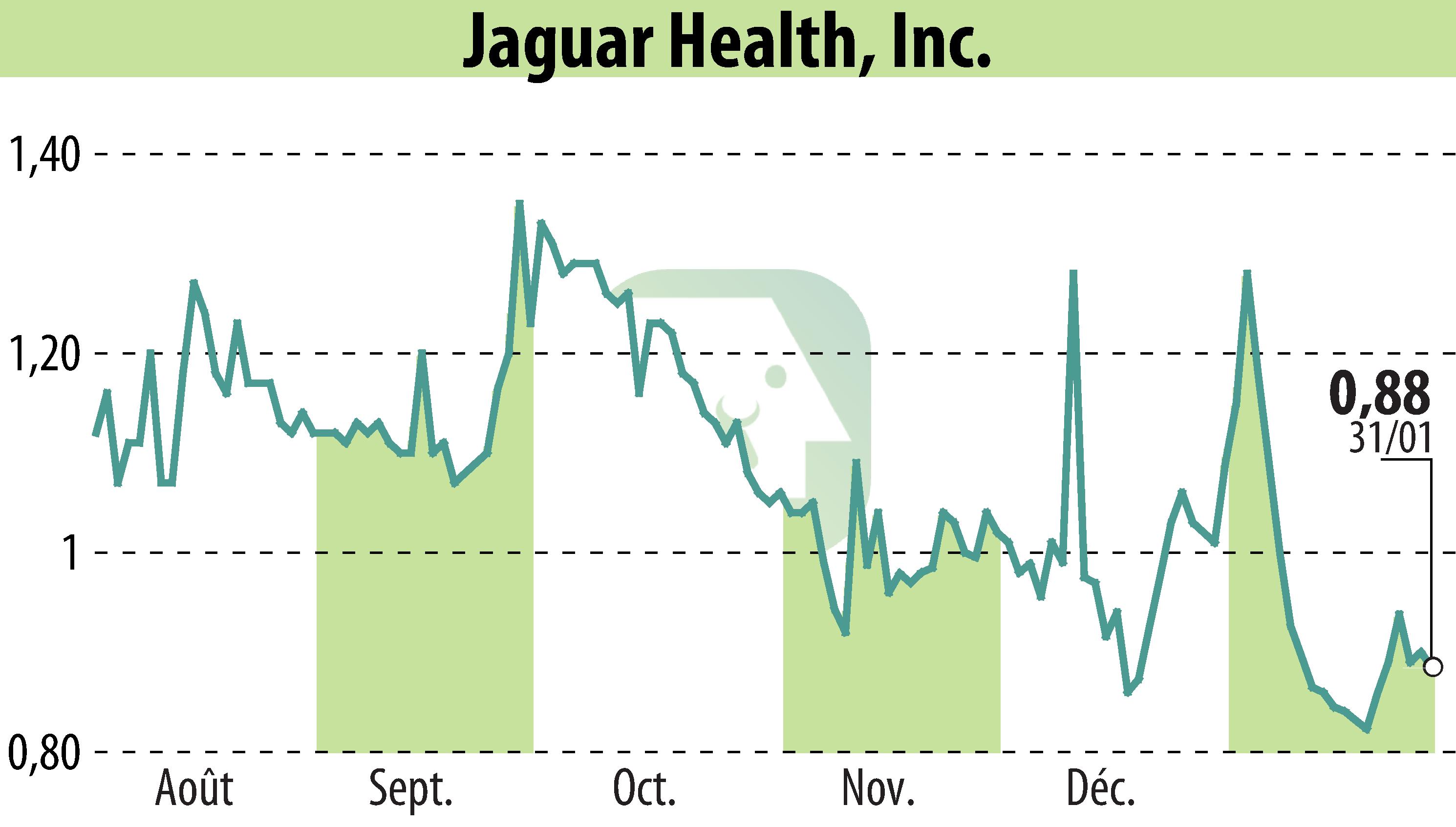
Jaguar Health, Inc. has begun dosing the first patient in a trial exploring crofelemer for Short Bowel Syndrome with Intestinal Failure (SBS-IF). This trial is hosted at Sheikh Khalifa Medical City in Abu Dhabi, led by Dr. Mohamad Miqdady. Crofelemer, a plant-based drug, has Orphan Drug Designation from the FDA and European Medicines Agency for both SBS-IF and Microvillus Inclusion Disease (MVID). Early trial results might lead to reimbursed access in European countries by mid-2025.
The trial addresses rare diseases requiring significant medical support such as SBS-IF and MVID. Both conditions entail serious health challenges, including severe diarrhea and malabsorption, making intensive parenteral nutrition necessary. Jaguar CEO Lisa Conte noted the trial as vital for crofelemer's development across various regions including the US and MENA.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
