sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Highlights Veterinary Support for Canalevia-CA1
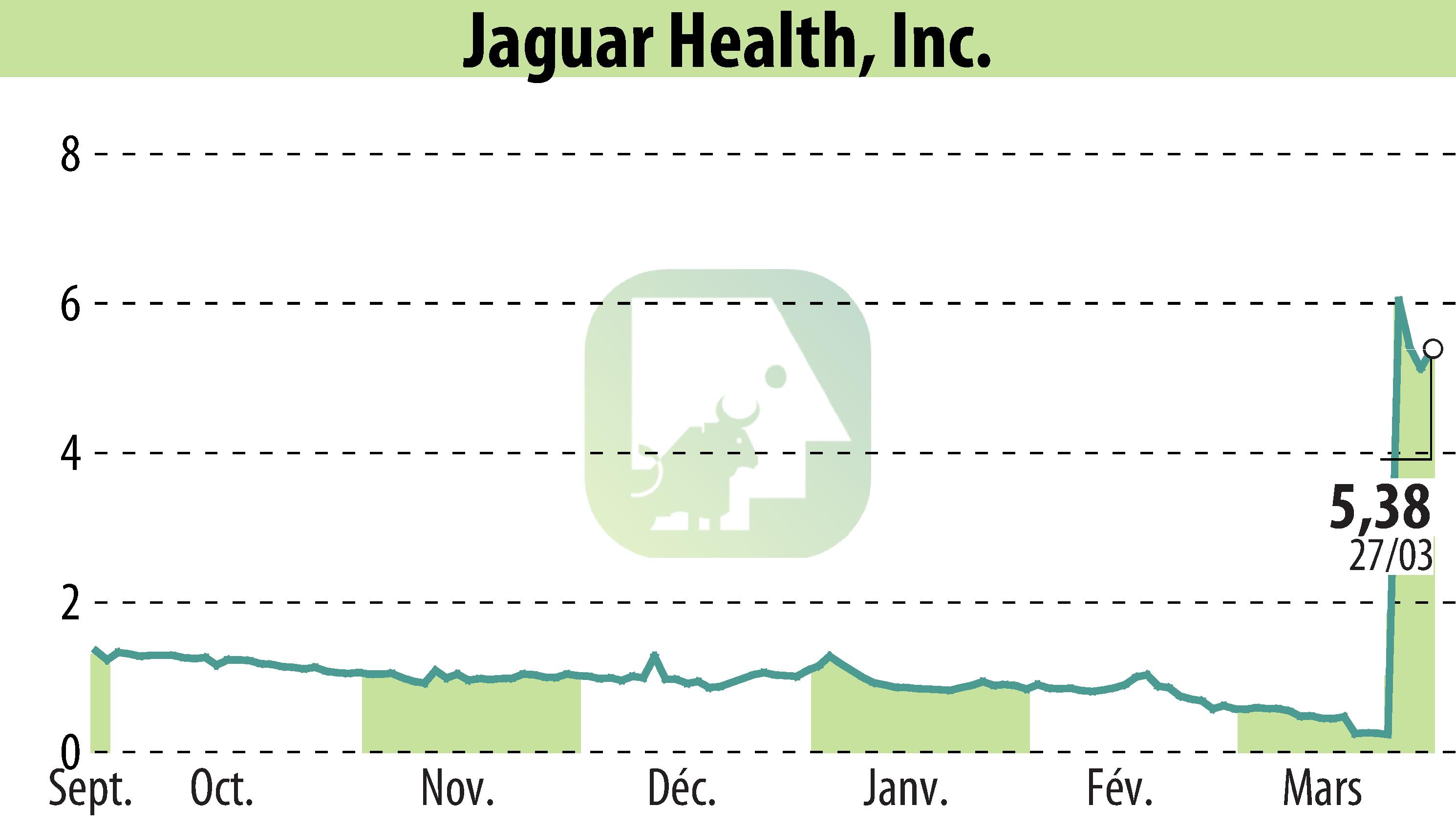
Jaguar Health, Inc. announced the findings of a survey among 27 U.S. veterinary oncologists regarding Canalevia-CA1. Canalevia-CA1 is an FDA conditionally approved treatment targeting chemotherapy-induced diarrhea (CID) in dogs. Of those surveyed, 83.33% highlighted the non-antimicrobial nature of Canalevia-CA1 as crucial due to concerns about antibiotic resistance.
Veterinarian Christine Swanson emphasized how Canalevia-CA1 allows cancer therapy continuation by managing gastrointestinal distress. This innovation provides an alternative to metronidazole, often used off-label for CID. The survey indicated that 62.5% of respondents avoid metronidazole due to antimicrobial resistance concerns.
Jaguar Health plans to collaborate with partners for further development of crofelemer, extending its application beyond CID to general non-infectious diarrhea in dogs.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
