sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Seeks EU Approval for Canalevia to Treat Canine Diarrhea
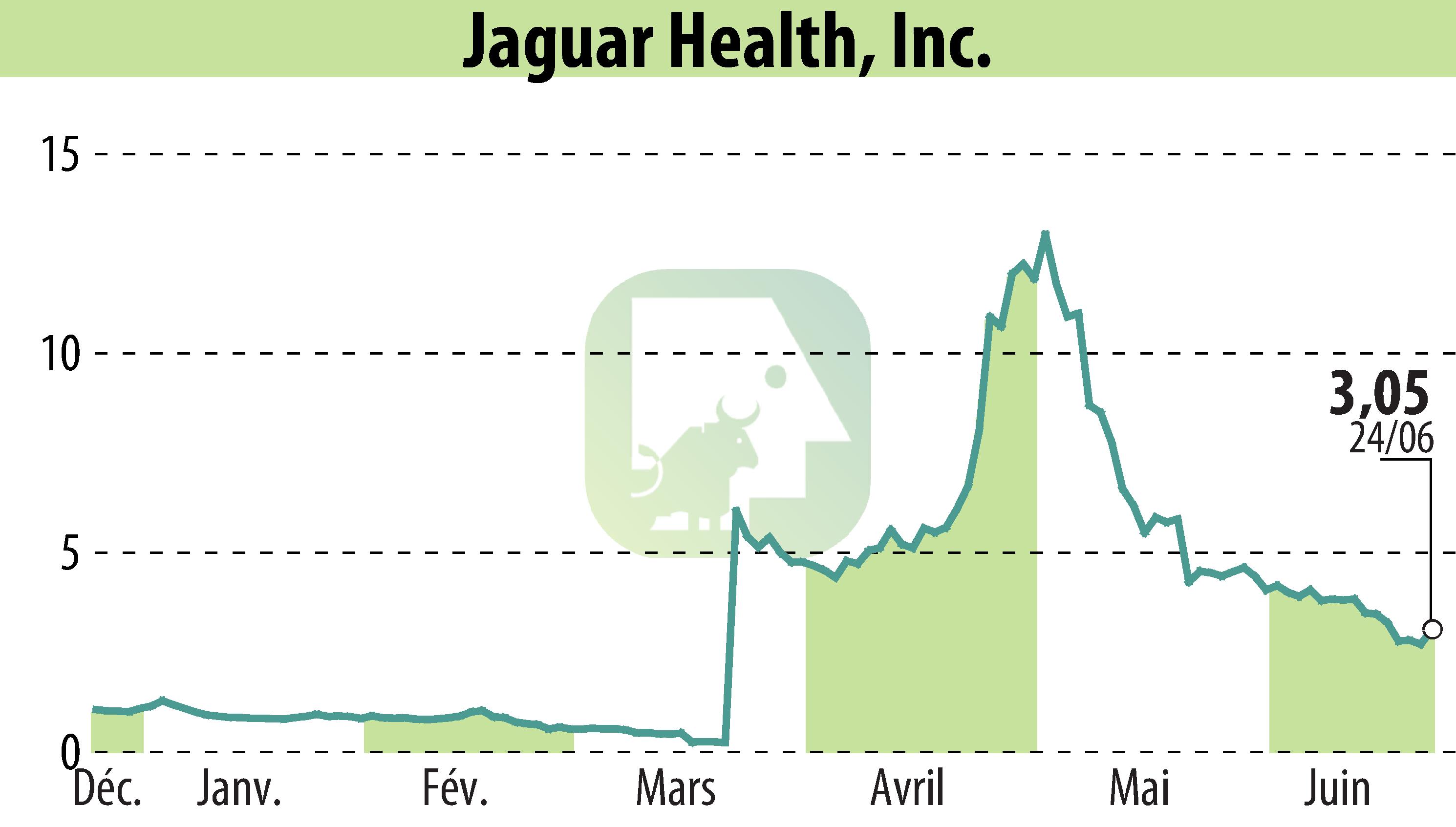
Jaguar Health, Inc. aims to gain approval in the European Union for Canalevia, a drug designed to treat general diarrhea in dogs. Already conditionally approved in the U.S. for chemotherapy-induced diarrhea, the company is now targeting a broader application. Despite not meeting initial endpoints, a study showed positive results for Canalevia in treating general diarrhea when re-evaluated, demonstrating better outcomes compared to placebos.
Jaguar Health is in discussions with potential partners to facilitate the global commercialization of Canalevia. The company plans to submit findings to the European Medicines Agency as a step toward seeking market authorization. If successful, the drug could address a significant medical need across the EU's 104 million dogs.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
