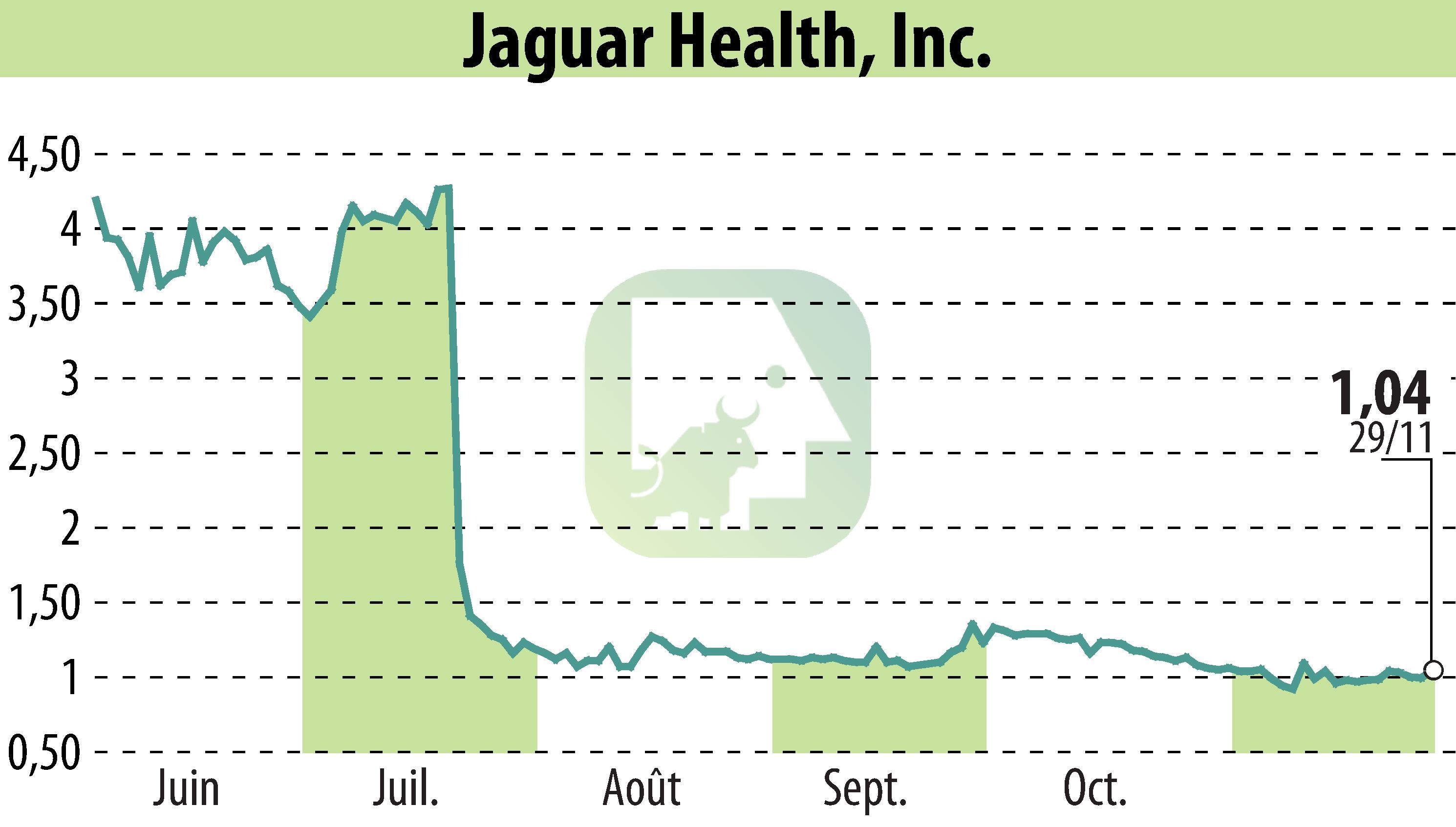
sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health Seeks Partner for Canine Diarrhea Drug at Pet Connect Conference
Jaguar Health, Inc. seeks a partner for the development and commercialization of NP300, a second-generation antisecretory drug for treating non-infectious diarrhea in dogs. This initiative will be highlighted at the Pet Connect Conference from December 3-5, 2024, in Los Angeles. The company aims to secure a partner to finance NP300’s development, offering U.S. commercial rights in return.
Diarrhea ranks as a top reason for veterinary visits, yet no FDA-approved treatment exists for general canine diarrhea. Jaguar’s existing drug, Canalevia®-CA1, is conditionally approved for treating chemotherapy-induced diarrhea in dogs. NP300, derived from the Croton lechleri tree, is noted for a similar mechanism and proven safety in dogs.
The U.S. veterinary market sees approximately six million annual cases of dog diarrhea, indicating strong demand for an effective treatment. With an estimated 89.7 million dogs in the U.S., addressing this need could significantly impact pet health care.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
