sur Jaguar Health, Inc. (NASDAQ:JAGX)
Jaguar Health's Crofelemer Shows Promise in Intestinal Failure Treatment
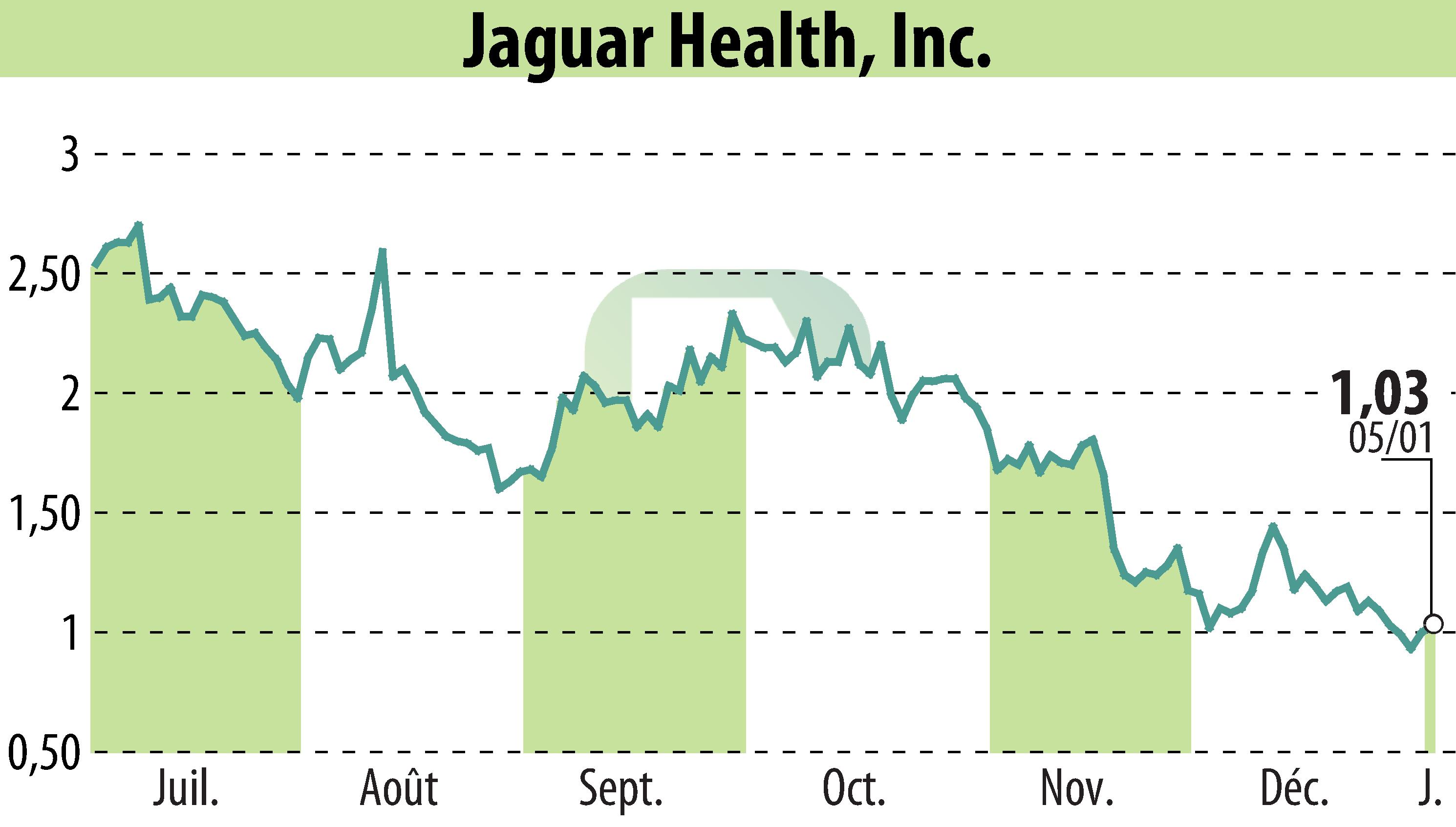
Jaguar Health, Inc. recently announced significant findings from a study on their drug, crofelemer, published in a UAE healthcare magazine. The proof-of-concept study demonstrated a reduction in parenteral support (PS) ranging from 12 to 37% for pediatric patients with intestinal failure due to conditions like short bowel syndrome (SBS-IF) and microvillus inclusion disease (MVID).
This reduction is crucial as PS is linked to severe toxicities and a lethal natural history. In the study, crofelemer lowered PS needs by up to 37% during an extension period for MVID patients. These findings may offer life-extending benefits to patients.
The study is part of a larger effort involving trials in the EU and the US, with crofelemer already gaining Orphan Drug Designation from regulatory authorities.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Jaguar Health, Inc.
