sur Moderna, Inc. (NASDAQ:MRNA)
Moderna Receives UK Approval for RSV Vaccine mRESVIA®
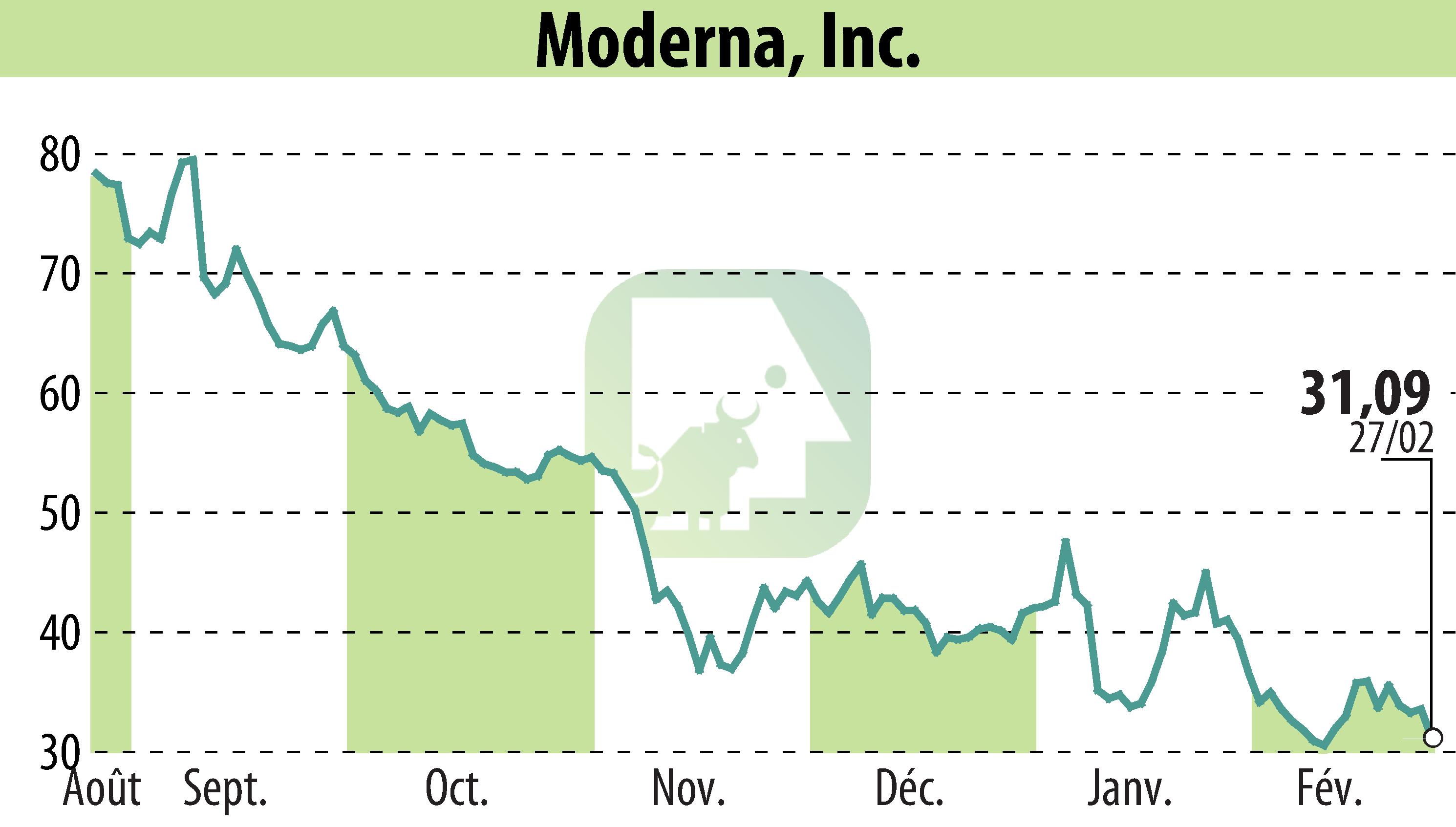
Moderna, Inc. has announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK has granted marketing authorization for its RSV vaccine, mRESVIA® (mRNA-1345), aimed at preventing lower respiratory tract disease in adults aged 60 and over. This marks Moderna's second approved product in the UK, signaling a significant step toward enhancing respiratory disease preparedness.
Respiratory syncytial virus (RSV) is a leading cause of lower respiratory infections and pneumonia among the elderly in the UK, contributing to numerous hospitalizations and deaths annually. The approval follows positive outcomes from the Phase 3 ConquerRSV trial, involving 37,000 participants worldwide, and showed no major safety issues.
Moderna's Oxfordshire site will manufacture the vaccine. This UK approval joins previous authorizations in the US, EU, Canada, and other regions. Moderna continues to seek regulatory approvals globally.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Moderna, Inc.
