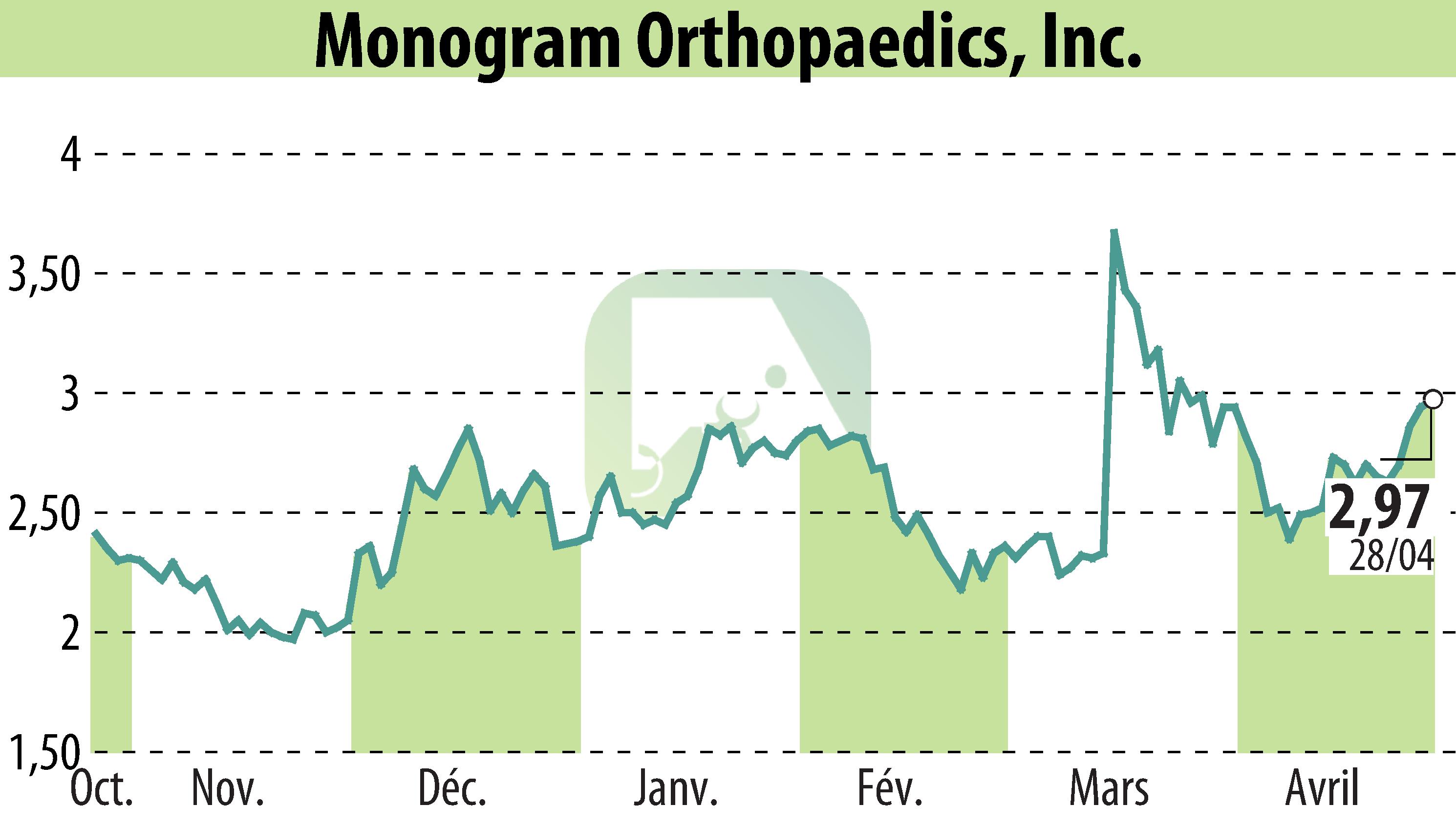
sur MONOGRAM ORTHOPAEDICS INC (NASDAQ:MGRM)
Monogram Technologies Obtains Approval for Clinical Trials in India
Monogram Technologies Inc. has received regulatory approval from India's Central Drugs Standard Control Organization to conduct a clinical investigation of its mBôs TKA system. This trial, in collaboration with Shalby Hospitals, will involve 102 patients and evaluates the system's safety and effectiveness. Patient enrollment is expected to begin shortly, with surgeries anticipated within 90 business days.
The study marks a significant milestone in the advancement of Monogram's AI-driven robotics in orthopedic surgery. By proving their technology in real surgical settings, Monogram aims to build clinical confidence and progress towards commercial launch. The company will also deploy full-time employees in India to ensure proper training and trial execution.
Key figures, including Dr. Vikram I. Shah, have expressed optimism about the trial's impact on orthopedic care innovation, positioning India as a leader in global medical advancements.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de MONOGRAM ORTHOPAEDICS INC
