sur Monogram Technologies Inc. (NASDAQ:MGRM)
Monogram Technologies Secures Approval for Clinical Trials in India
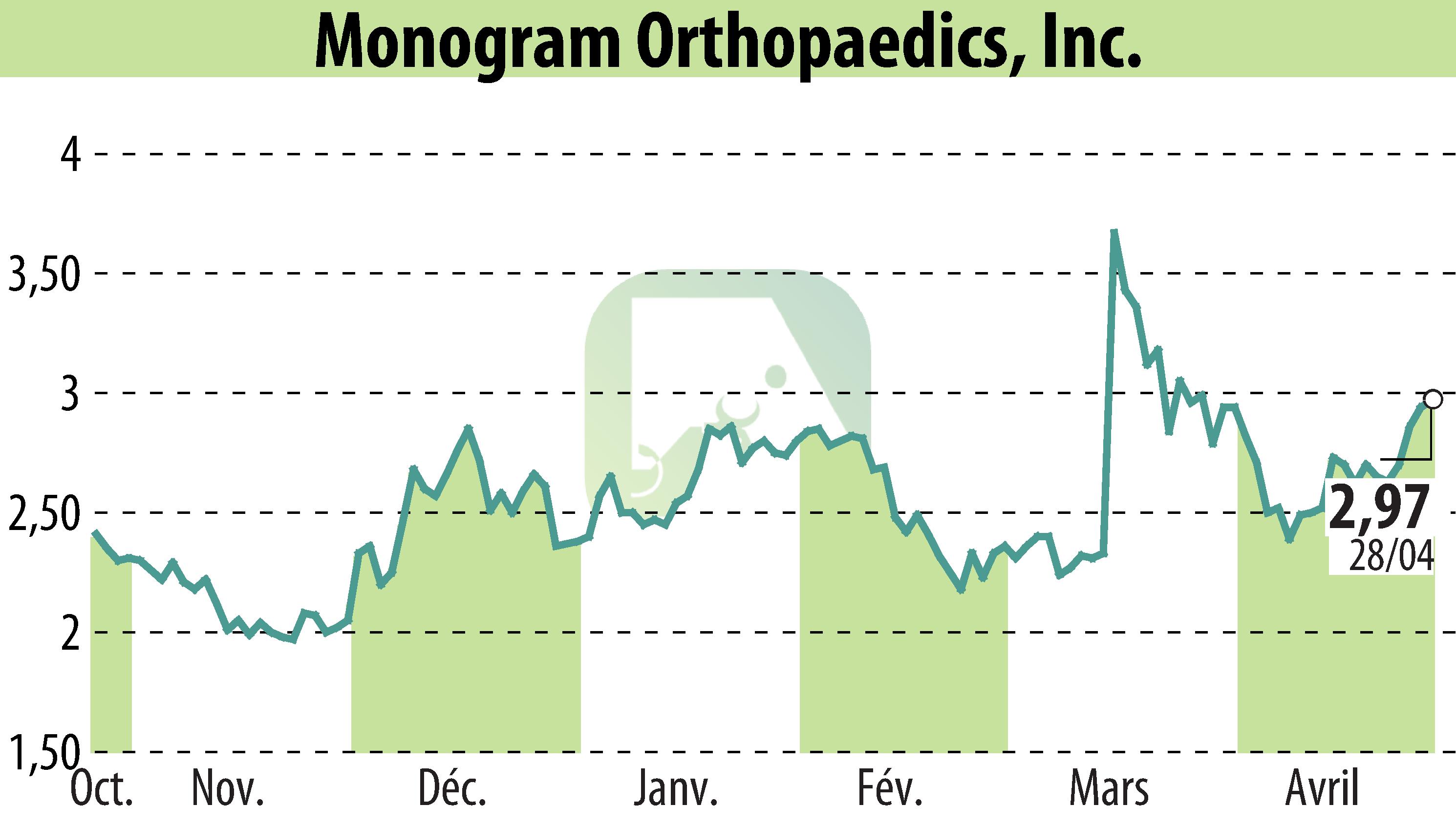
Monogram Technologies Inc., an AI-driven robotics company, has received regulatory permission from India's CDSCO to import and test its mBôs TKA system. This approval marks the start of a 102-patient clinical trial in partnership with Shalby Hospitals, targeting the efficacy of the system for knee replacement surgery. The trial will be spread over multiple centers in India, with patient enrollment to begin soon and surgeries expected within 90 days.
This collaboration represents a significant milestone in orthopedic healthcare, involving the robotic mBôs TKA System paired with Consensus CKS implants. Training and preparations for the trial have been ongoing, with Monogram positioning staff in India to assist. Leaders from Monogram and Reliance Life Sciences emphasize the trial’s potential to advance orthopedic innovation and improve surgical outcomes.
The project's execution aligns with Monogram's dedication to enhancing patient care through pioneering technologies in orthopedic surgery, which could set new standards of care globally.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Monogram Technologies Inc.
