sur NANOBIOTIX (EPA:NANO)
NANOBIOTIX Reports Promising Phase 1 Results for JNJ-1900 (NBTXR3) in R/M-HNSCC
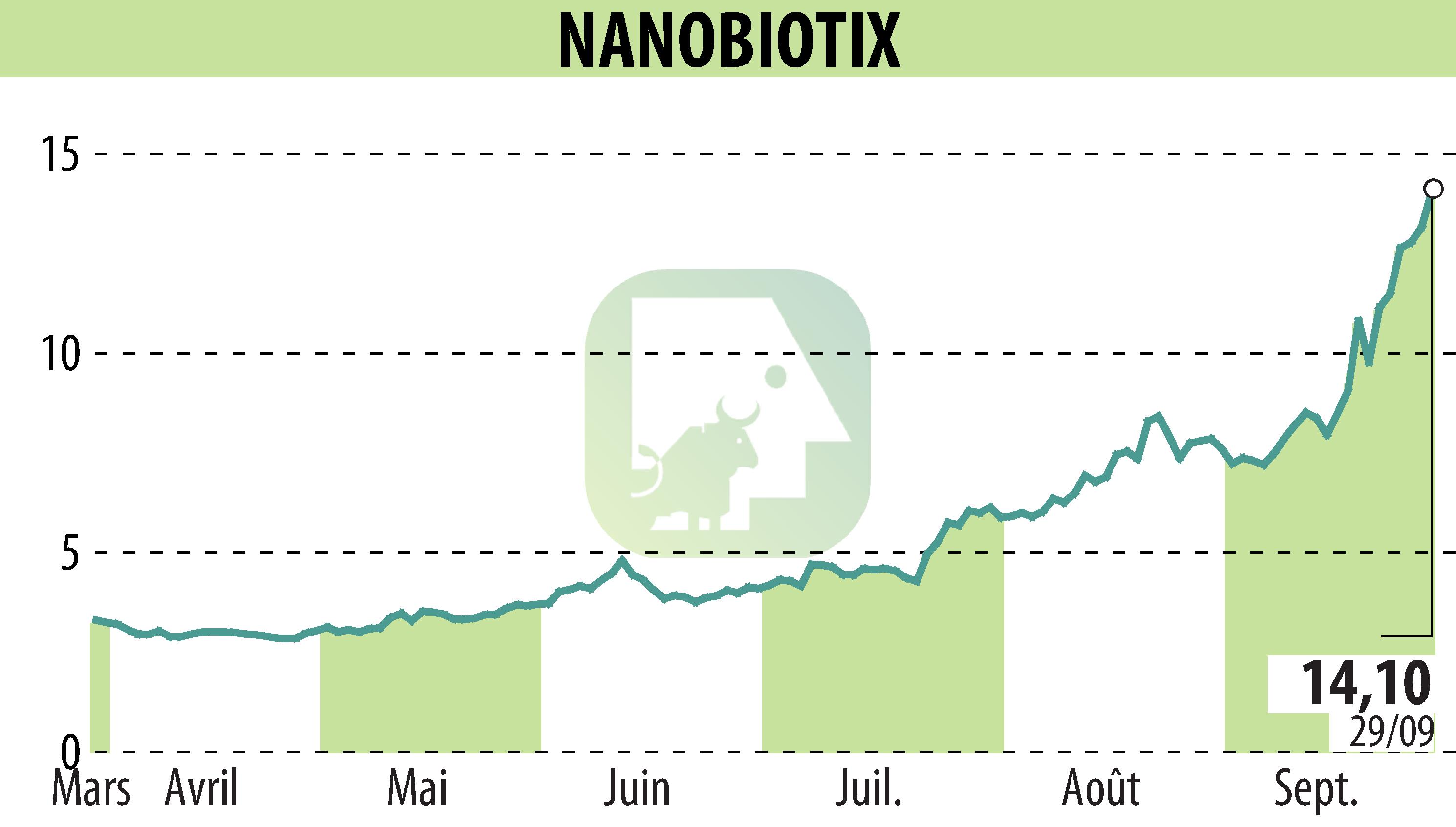
NANOBIOTIX has announced updated data from its Phase 1 trial of JNJ-1900 (NBTXR3) combined with anti-PD-1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma (R/M-HNSCC) at the ASTRO 2025 Annual Meeting. The study included anti-PD-1 naïve and resistant cohorts, showing encouraging disease control (DCR) and overall response rates (ORR).
The trial revealed that JNJ-1900, activated by radiotherapy, was well-tolerated and feasible in heavily pre-treated patients, maintaining high injected-lesion control and response rates. Specifically, anti-PD-1 naïve patients displayed a 95% DCR and a 66% ORR, while anti-PD-1 resistant patients showed a 94% DCR and a 50% ORR.
The median overall survival (OS) reached 15.5 months in anti-PD-1 naïve patients and 11.4 months in resistant patients, suggesting a potential to overcome prior immune checkpoint inhibitor resistance. These promising outcomes underscore the need for further exploration in randomized studies.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de NANOBIOTIX
