sur Nanohale AG (ETR:FYB)
Formycon Partners with NTC for Biosimilar Commercialization in Italy
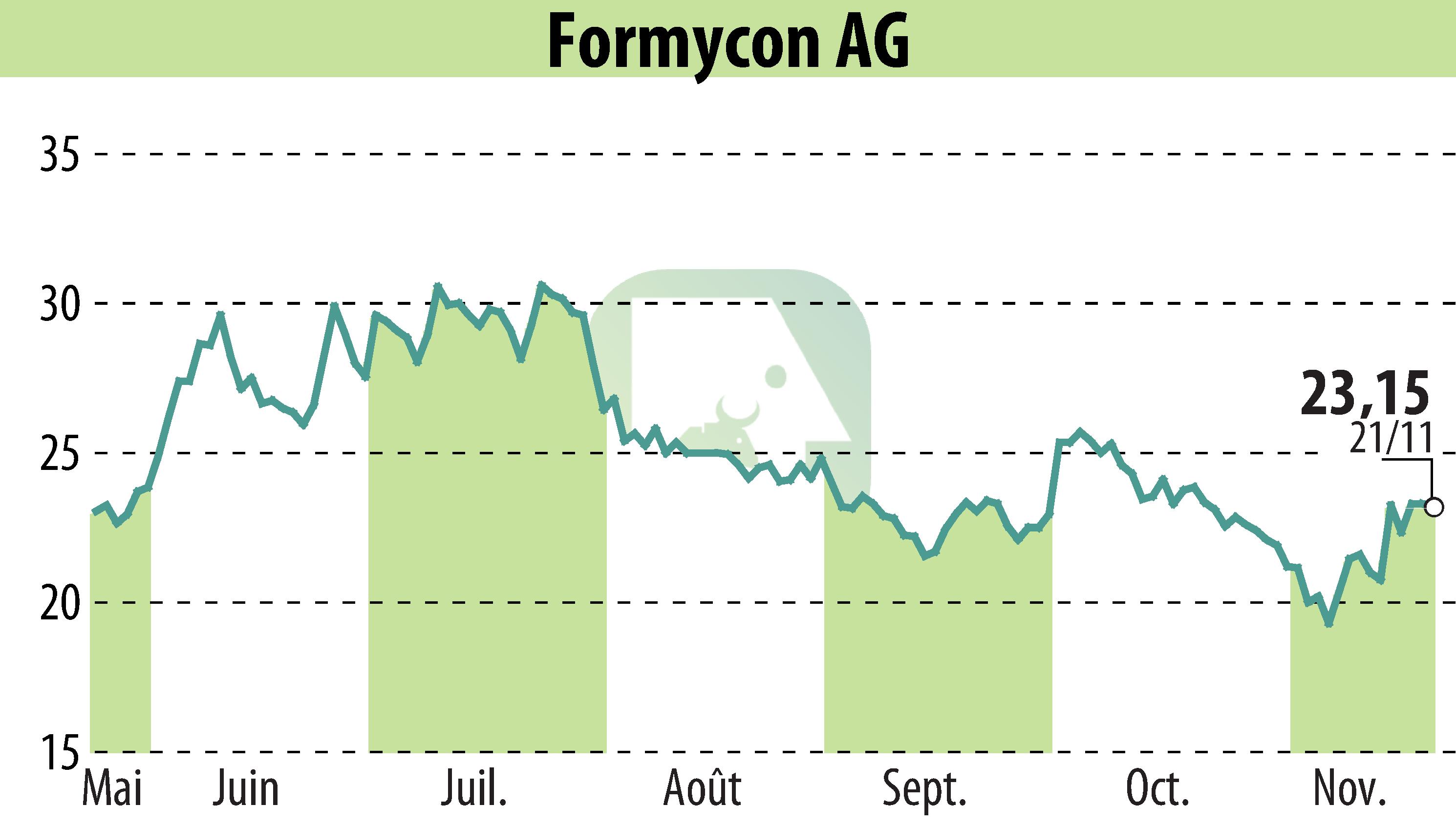
Formycon AG, a German developer of biosimilars, has announced a partnership with Italy-based NTC s.r.l. for the commercialization of its Eylea® biosimilar, FYB203/Baiama®, in Italy. Klinge Biopharma GmbH, which holds global commercialization rights, finalized this exclusive agreement. This move allows Formycon to organize the supply chain, earning service payments and a volume-based profit segment.
The decision aligns with the growing demand for treatments for age-related diseases such as wet macular degeneration in an aging European population. The FYB203 biosimilar offers a cost-effective alternative for these conditions. NTC, known for its expertise in ophthalmology, will leverage its market knowledge and customer networks for distribution.
FYB203 received FDA approval in June 2024 and European Commission approval in January 2025. This biosimilar inhibits VEGF, combating diseases leading to excessive blood vessel formation in the retina.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Nanohale AG
