sur Nanohale AG (ETR:FYB)
Lucentis® Biosimilar FYB201/Ranivisio® Gains Approval in Brazil
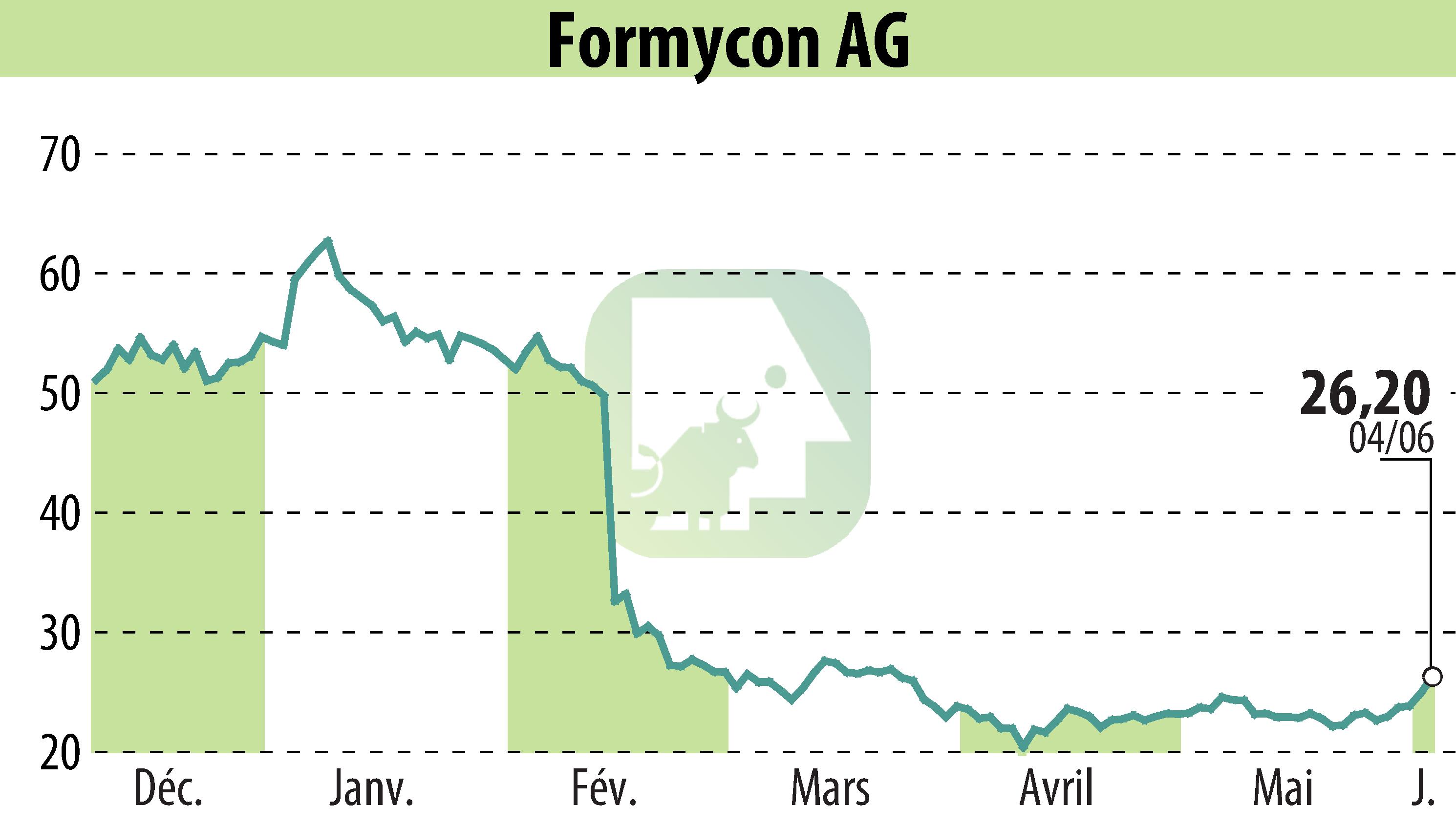
Formycon AG, Germany, has announced that the biosimilar FYB201/Ranivisio®, equivalent to Lucentis® (ranibizumab), has received marketing authorization from Brazil's regulatory authority, ANVISA. Ranivisio® marks the first approved Lucentis® biosimilar in Brazil, set to launch in Q4 2025 in partnership with Biomm, a Brazilian pharmaceutical firm, marking the beginning of its rollout across Latin America.
Market authorizations have already been acquired in Peru, El Salvador, Honduras, and the Dominican Republic. Further launches in Central and South America are anticipated through early 2027. Biomm, well-established in Brazil's healthcare market, will leverage its local expertise to drive commercialization. The Brazilian anti-VEGF therapies market is valued at BRL 374 million annually.
FYB201/Ranivisio® addresses severe retinal diseases, developed through Bioeq AG, a collaboration between Formycon and Polpharma Biologics Group. The biosimilar is accessible across 21 countries, inclusive of regions in Europe, North America, and MENA.
R. E.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Nanohale AG
