sur SANOFI-AVENTIS (EPA:SAN)
Sanofi and Regeneron's Dupixent Gains Approval in Japan for Young Asthma Patients
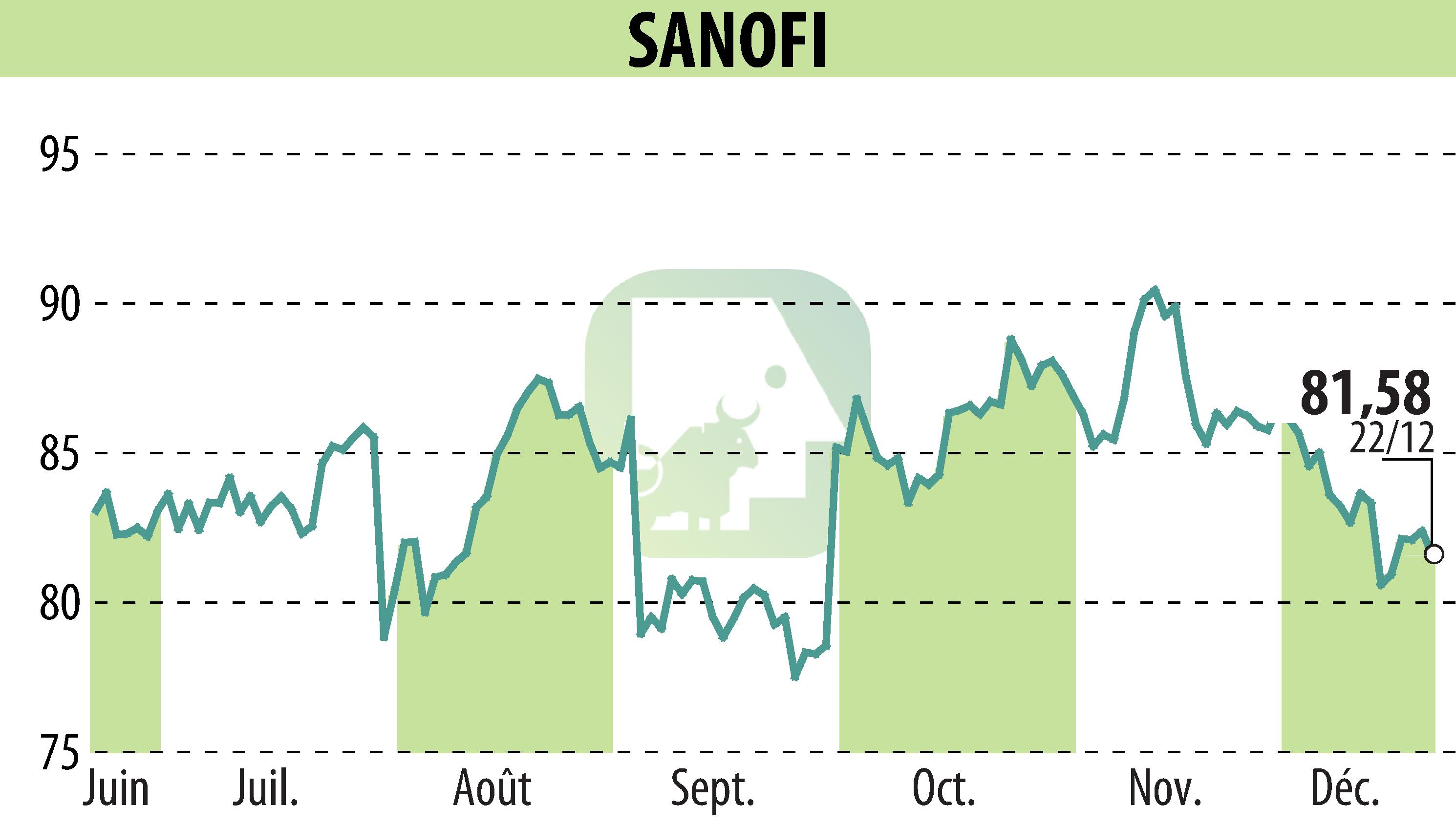
Sanofi and Regeneron have announced that their biologic medication Dupixent has received approval from Japan's Ministry of Health, Labour and Welfare for use in children aged 6 to 11 with severe bronchial asthma. This decision expands the previous authorization for patients aged 12 and above.
The approval is grounded on global phase 3 studies demonstrating Dupixent's efficacy. The trials showed a significant reduction in asthma exacerbations and improved lung function in this young demographic.
Dupixent targets type 2 inflammation by inhibiting interleukin-4 and interleukin-13, pivotal in asthma's pathology. Common side effects include injection site reactions and mild systemic symptoms.
Beyond asthma, Dupixent is approved for multiple conditions in Japan, reinforcing its therapeutic versatility. This development further marks the drug's availability across 50 countries for pediatric asthma treatment.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
