sur SANOFI-AVENTIS (EPA:SAN)
Sanofi obtains approval in China for Qfitlia and Cablivi
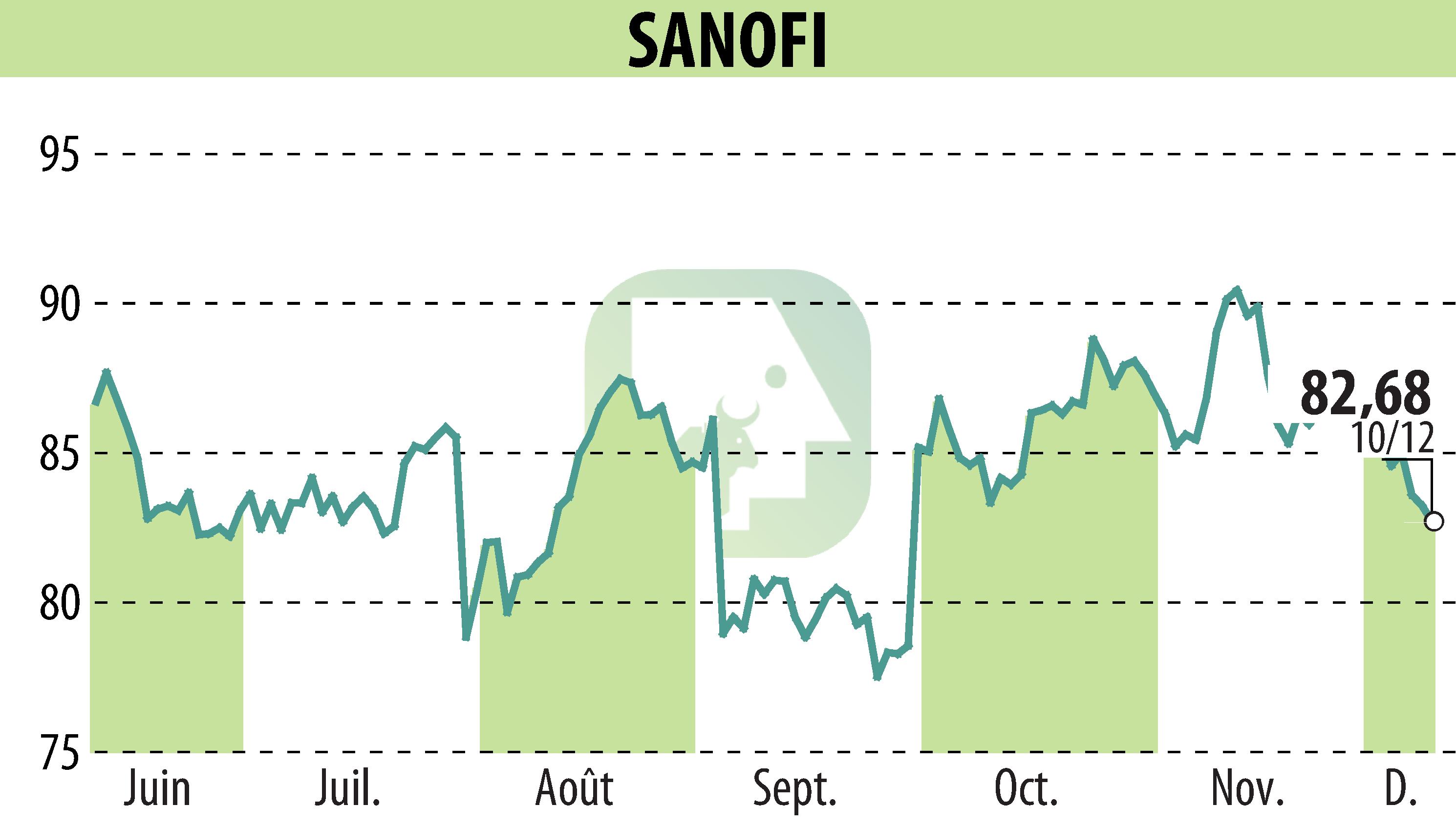
Sanofi has announced the approval of Qfitlia and Cablivi in China by the National Medical Products Administration. These two drugs, intended for rare hematological diseases, reinforce Sanofi's commitment to the Chinese market. Qfitlia, a treatment for hemophilia, is distinguished by its simplified administration: only six injections per year. It has demonstrated its efficacy in the ATLAS studies, significantly reducing bleeding. Cablivi, on the other hand, targets acquired thrombotic thrombocytopenic purpura, a rare and potentially fatal disorder. It works by inhibiting the interaction between von Willebrand factor and platelets, thus reducing the risk of organ damage. These innovations demonstrate Sanofi's commitment to providing medical solutions to critical needs in the field of rare diseases.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
