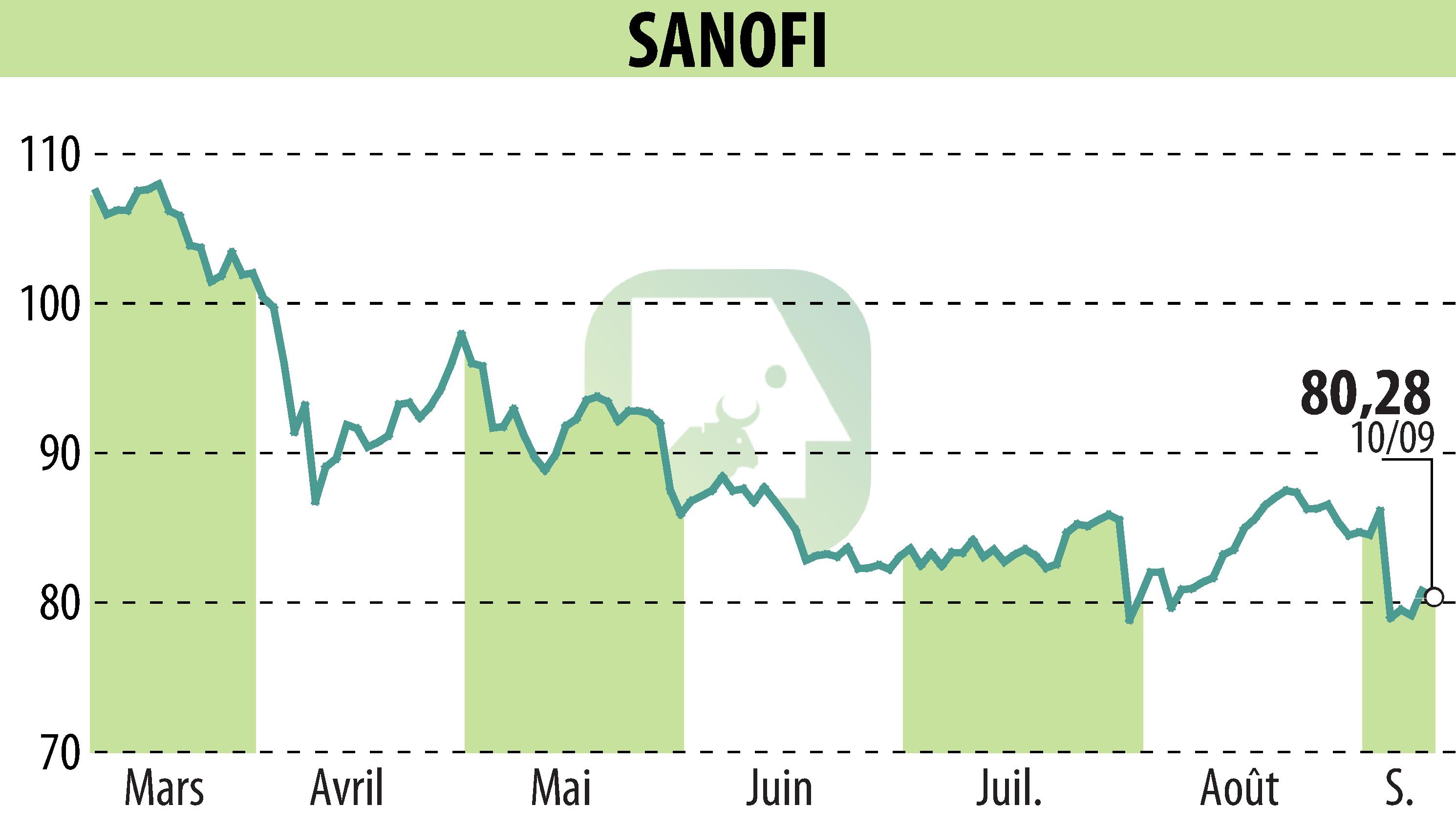
sur SANOFI-AVENTIS (EPA:SAN)
Sanofi Obtains Fast Track Designation for SAR402663 in the United States
Sanofi announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to its product SAR402663. This innovative gene therapy is intended for the treatment of neovascular age-related macular degeneration (AMD). This serious condition can lead to significant vision loss, affecting more than one million Americans. The designation will facilitate and expedite the development and review of this drug, designed to inhibit the abnormal growth of blood vessels in the eye.
Currently in Phase I/II clinical trials, Sanofi is evaluating the efficacy of SAR402663 in reducing vascular leakage and retinal damage. This initiative is part of the company's efforts to address unmet medical needs and improve patient quality of life. With a single administration, the treatment could replace repeated intravitreal injections, simplifying patient care.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
