sur SANOFI-AVENTIS (EPA:SAN)
Sanofi's SAR446268 Therapy Advances in the United States
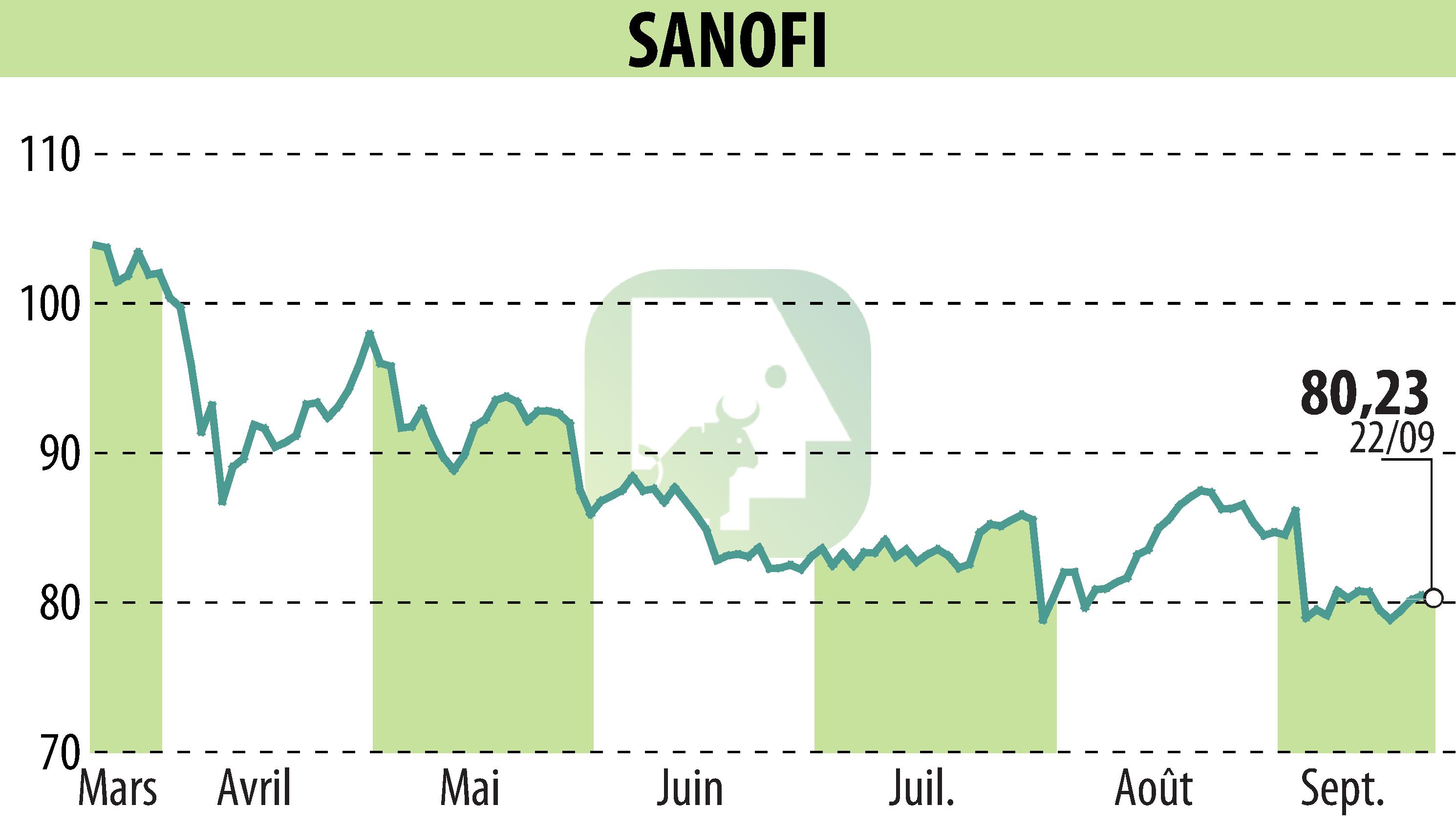
Sanofi announced that its gene therapy SAR446268 has been granted fast-track approval by the U.S. FDA. This decision concerns the treatment of myotonic dystrophy type 1 (DM1), a rare genetic disorder that causes muscle weakness and wasting. Currently, no medication is approved for this condition.
SAR446268 uses vector-based RNA interference to target and silence DMPK gene expression. This novel approach could improve muscle function by restoring normal tissue splicing.
The treatment is in phase 1-2 clinical trials to assess its safety and efficacy. Sanofi, which has already been granted orphan status in the United States and the EU, aims to address an unmet medical need.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
