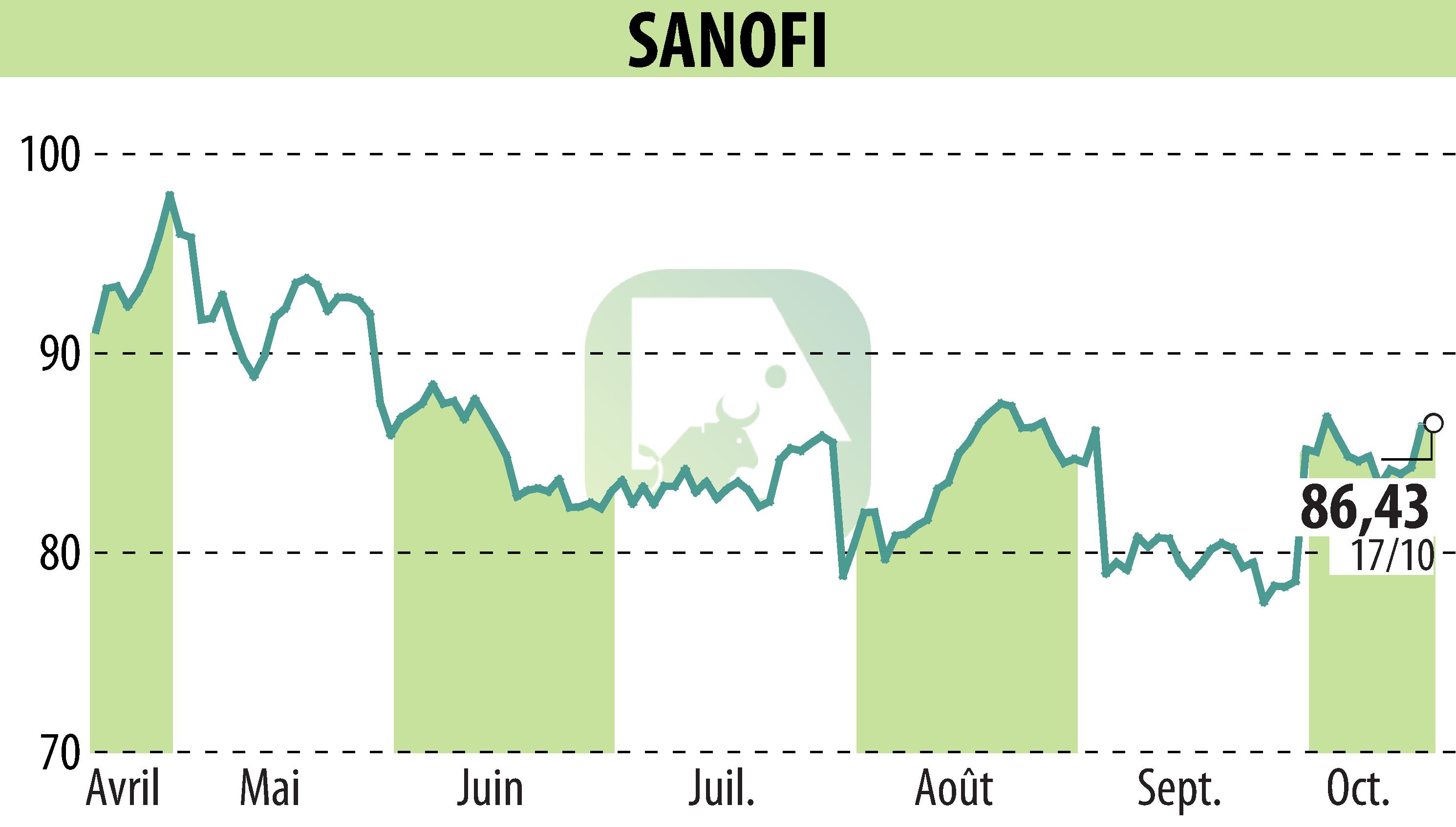
sur SANOFI-AVENTIS (EPA:SAN)
Sanofi's Tzield: FDA Fast-Tracks Review for Type 1 Diabetes
Sanofi announced that its treatment, Tzield, has had its Biologics License Application accepted for expedited review by the FDA. This drug aims to slow the progression of stage 3 type 1 diabetes in adults and children eight years of age and older who have been recently diagnosed. Tzield could become the first disease-modifying therapy in this area.
Tzield's acceptance under the Commissioner's National Priority Voucher underscores its potential in medicine, addressing unmet medical needs. The FDA could reduce the typical review time from 10 to 12 months to just one to two months. This would allow Sanofi to bring this treatment to patients more quickly.
The results of the PROTECT study, supporting this application, show significant preservation of beta cell function compared to placebo. Tzield is already approved in several countries to delay the onset of advanced type 1 diabetes.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
