sur SANOFI-AVENTIS (EPA:SAN)
Sanofi's Wayrilz recommended by CHMP for IPT
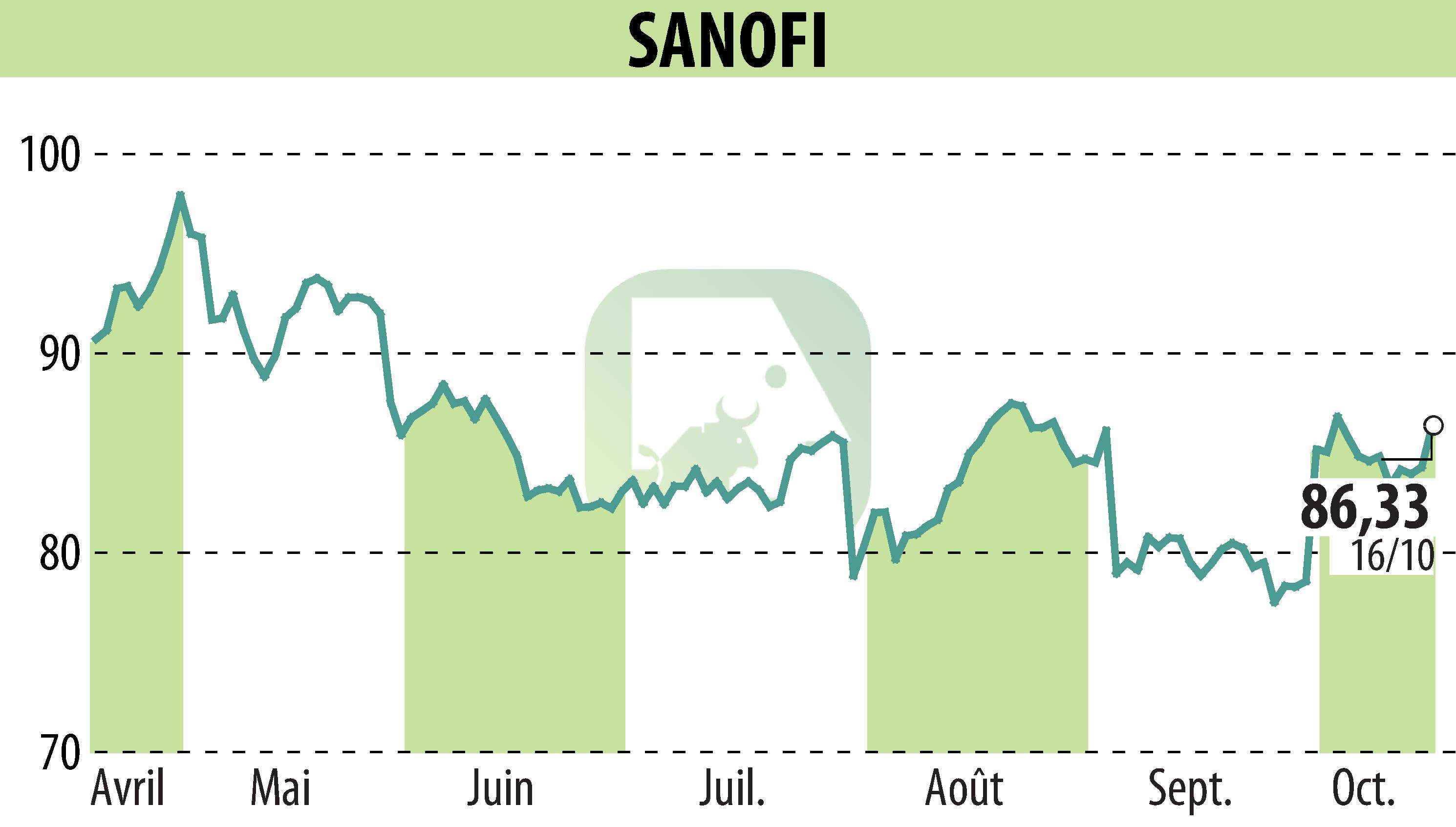
Wayrilz, Sanofi's treatment for immune thrombocytopenia (ITP), has received a positive recommendation from the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP). This decision follows results from the Phase 3 LUNA 3 study, which demonstrated the efficacy of Wayrilz in terms of platelet response and improvement in ITP symptoms.
If approved, Wayrilz will become the first BTK inhibitor to treat IPD in the European Union, targeting the immune dysregulation that causes the disease through multi-immune modulation.
The treatment, already approved in the United States and the United Arab Emirates, is also under review in China. In addition to IPT, Wayrilz is being studied for other rare diseases such as autoimmune hemolytic anemia.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
