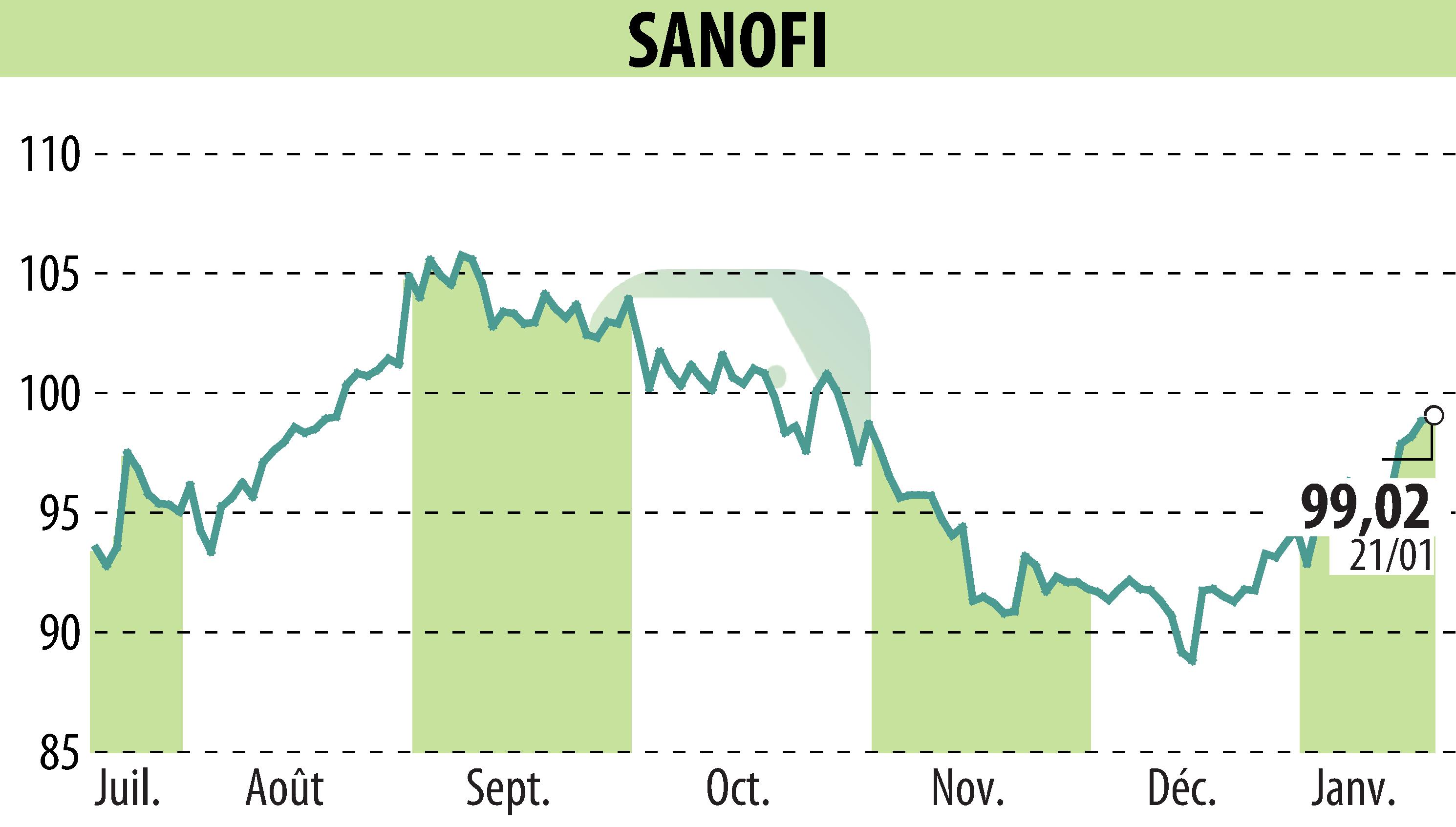
sur SANOFI-AVENTIS (EPA:SAN)
Sarclisa approved in EU for treatment of multiple myeloma
Sanofi-Aventis announced that the European Commission has approved Sarclisa for the treatment of newly diagnosed multiple myeloma in adults who are not eligible for transplantation. This decision follows a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) and is based on positive results from the IMROZ Phase III study.
Sarclisa, in combination with VRd (bortezomib, lenalidomide and dexamethasone), thus becomes the first anti-CD38 approved for this specific use in the European Union. This approval offers a new option for patients for whom current alternatives are limited, addressing a significant medical need.
This advancement follows the approval of the US FDA in September 2024, where Sarclisa was also granted orphan drug status for this indication.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
