sur SANOFI-AVENTIS (EPA:SAN)
Sarclisa Gains Approval in Japan for Multiple Myeloma Treatment
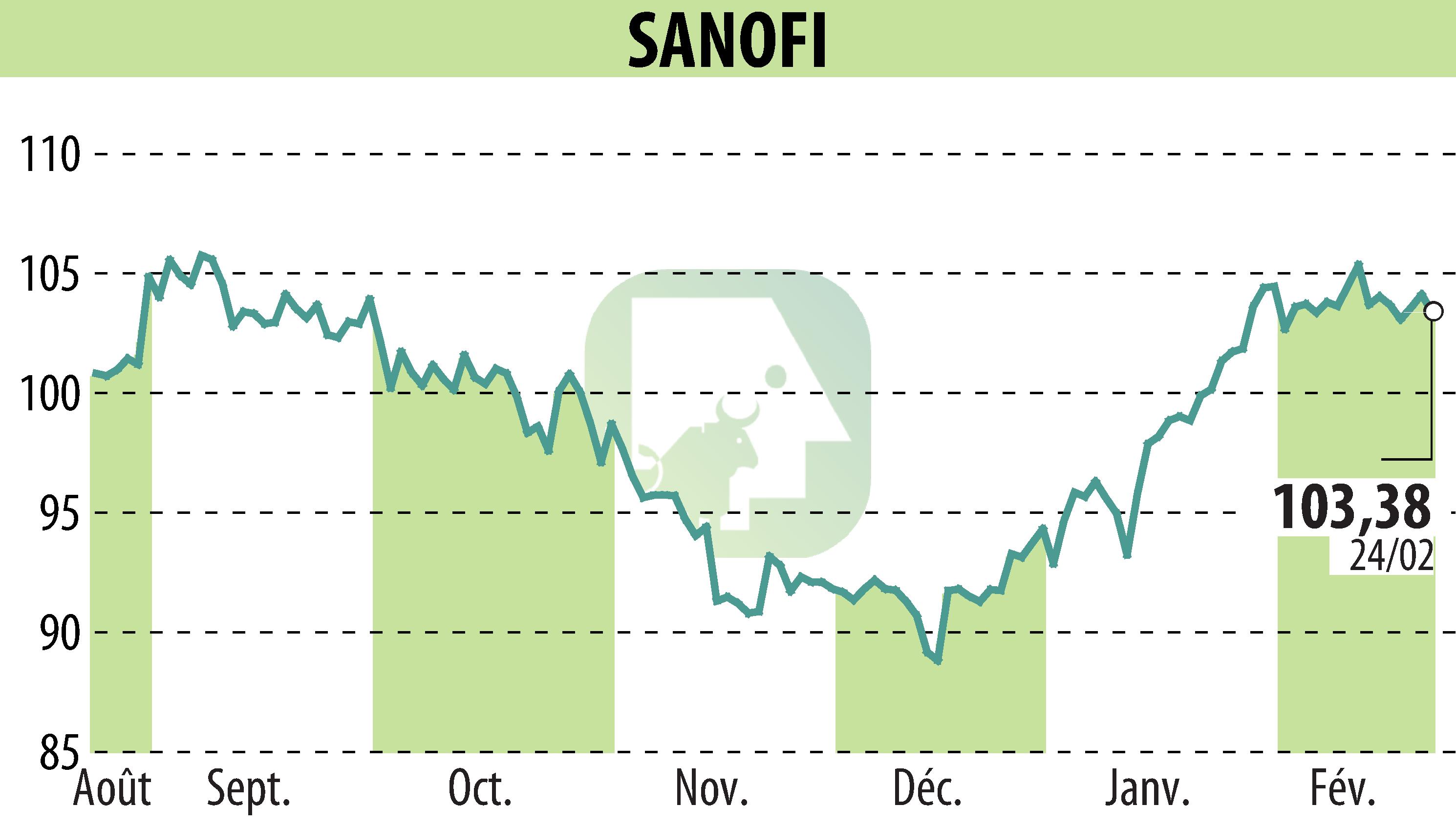
The Japanese Ministry of Health, Labour and Welfare has approved Sarclisa, in combination with VRd, for treating newly diagnosed multiple myeloma patients. This decision is founded on positive results from the IMROZ phase 3 study, which highlighted Sarclisa's significant impact on progression-free survival compared to VRd alone.
Olivier Nataf, Global Head of Oncology at Sanofi, highlighted the growing incidence of multiple myeloma in Japan and the need for novel treatments. This approval marks the first for Sarclisa in newly diagnosed patients in Japan, further extending its reach as a front-line treatment in Japan, the EU, and the US.
Globally, Sarclisa is approved in over 50 countries and is currently under evaluation in several clinical trials, including studies exploring a new subcutaneous administration method. Sanofi emphasizes its ongoing commitment to advancing treatment options for challenging cancers.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de SANOFI-AVENTIS
