sur Tharimmune Inc. (NASDAQ:THAR)
Tharimmune Receives FDA Nod for TH104 NDA Pathway
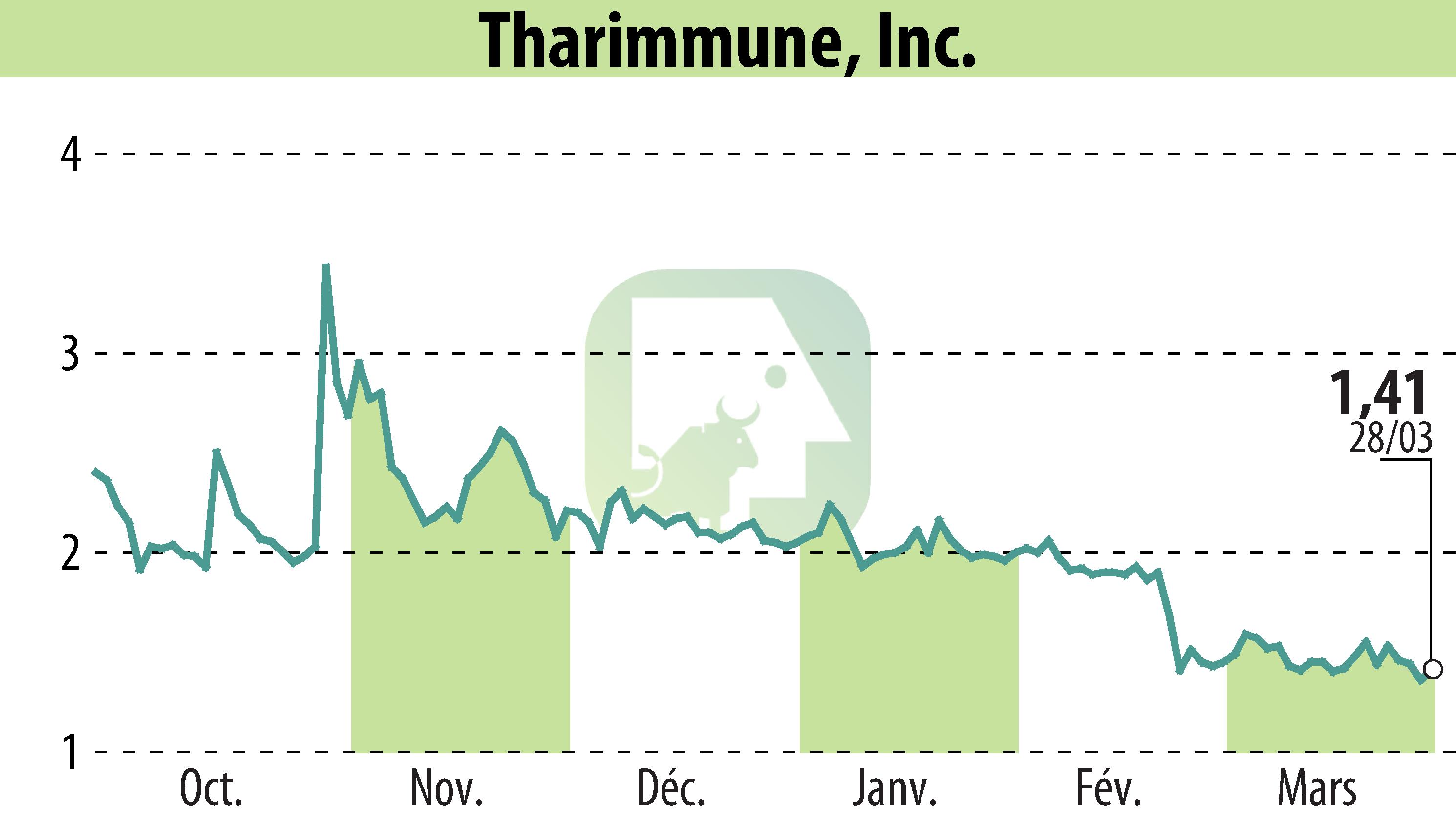
Tharimmune, Inc., a biotechnology firm based in Bridgewater, NJ, announced positive feedback from the FDA on its plan to submit a New Drug Application for TH104. Utilizing the 505(b)(2) pathway, this application does not require additional clinical trials, thanks to existing pharmacokinetic data.
TH104, which incorporates nalmefene, aims to prevent respiratory and nervous system depression due to potent opioid exposure. This product also benefits from its buccal film formulation, offering rapid protection. Randy Milby, CEO, highlights the significance of these advancements in enhancing prophylactic measures for military personnel and responders.
The submission plan leverages prior data and sophisticated modeling to predict drug performance, focusing on Chemistry, Manufacturing, and Controls (CMC) to ensure product quality. Further updates from Tharimmune will be accessible through their investor relations page.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de Tharimmune Inc.
