sur VALNEVA (EPA:VLA)
Valneva obtains positive results in Phase 2 for the chikungunya vaccine in children
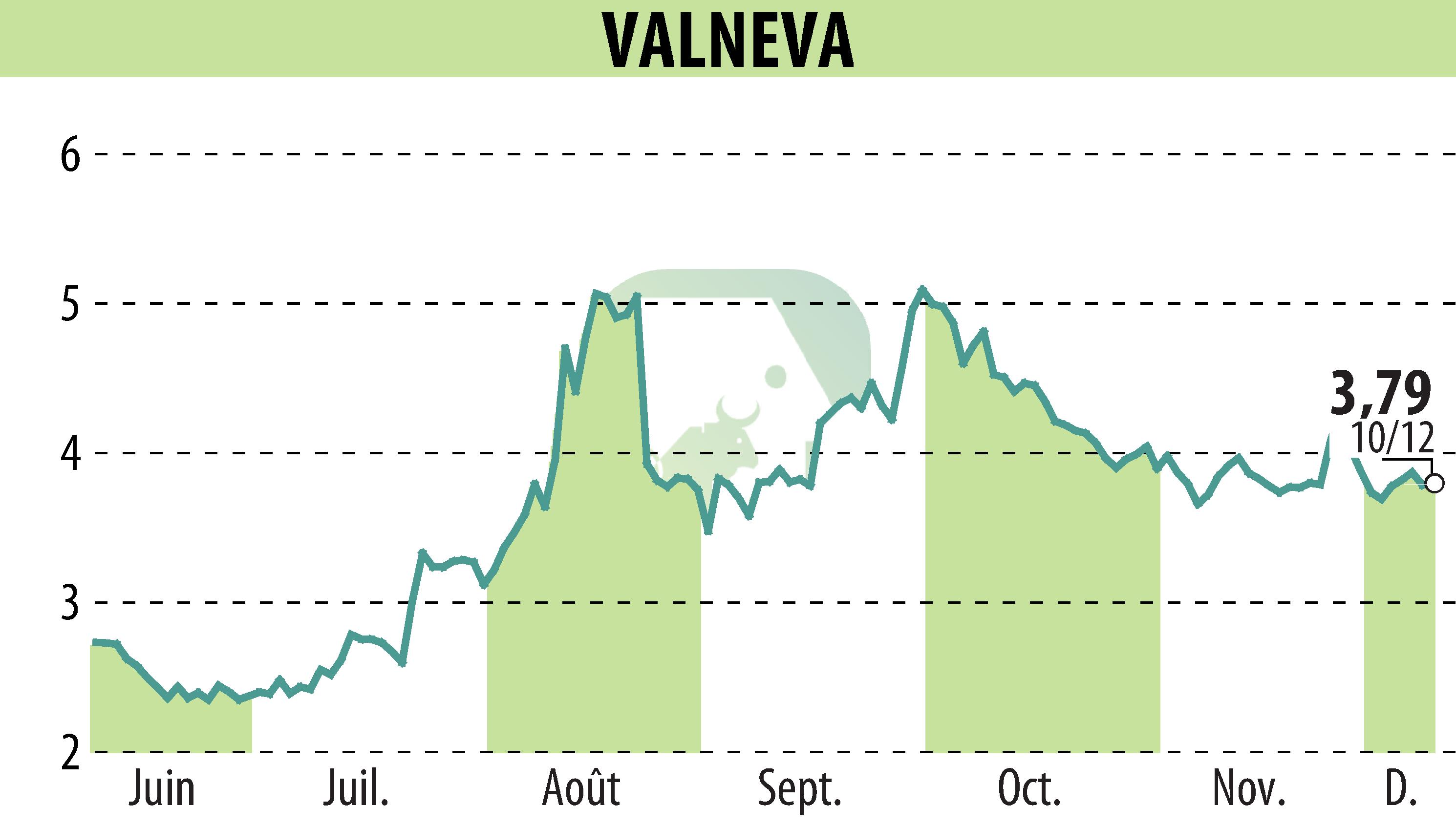
Valneva announced positive final Phase 2 results regarding the safety and antibody persistence of its chikungunya vaccine, IXCHIQ®, in children. The study, conducted with 304 children, demonstrated that the vaccine is well tolerated, regardless of dose or prior infection.
Twelve months after vaccination, antibody levels remain high, particularly with a full dose. These results support the decision to proceed to a Phase 3 trial. The trial was supported by the Coalition for Epidemic Preparedness Innovations and the European Union. Valneva plans to launch a future Phase 3 pediatric study.
Brazil and India report the majority of cases, highlighting the importance of globally accessible vaccination. Valneva, in partnership with regulatory authorities, continues its efforts to make the vaccine available to all age groups, especially in low-income countries.
R. H.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de VALNEVA
