sur VALNEVA (EPA:VLA)
Valneva Updates on Chikungunya Vaccine Recommendation in the U.S.
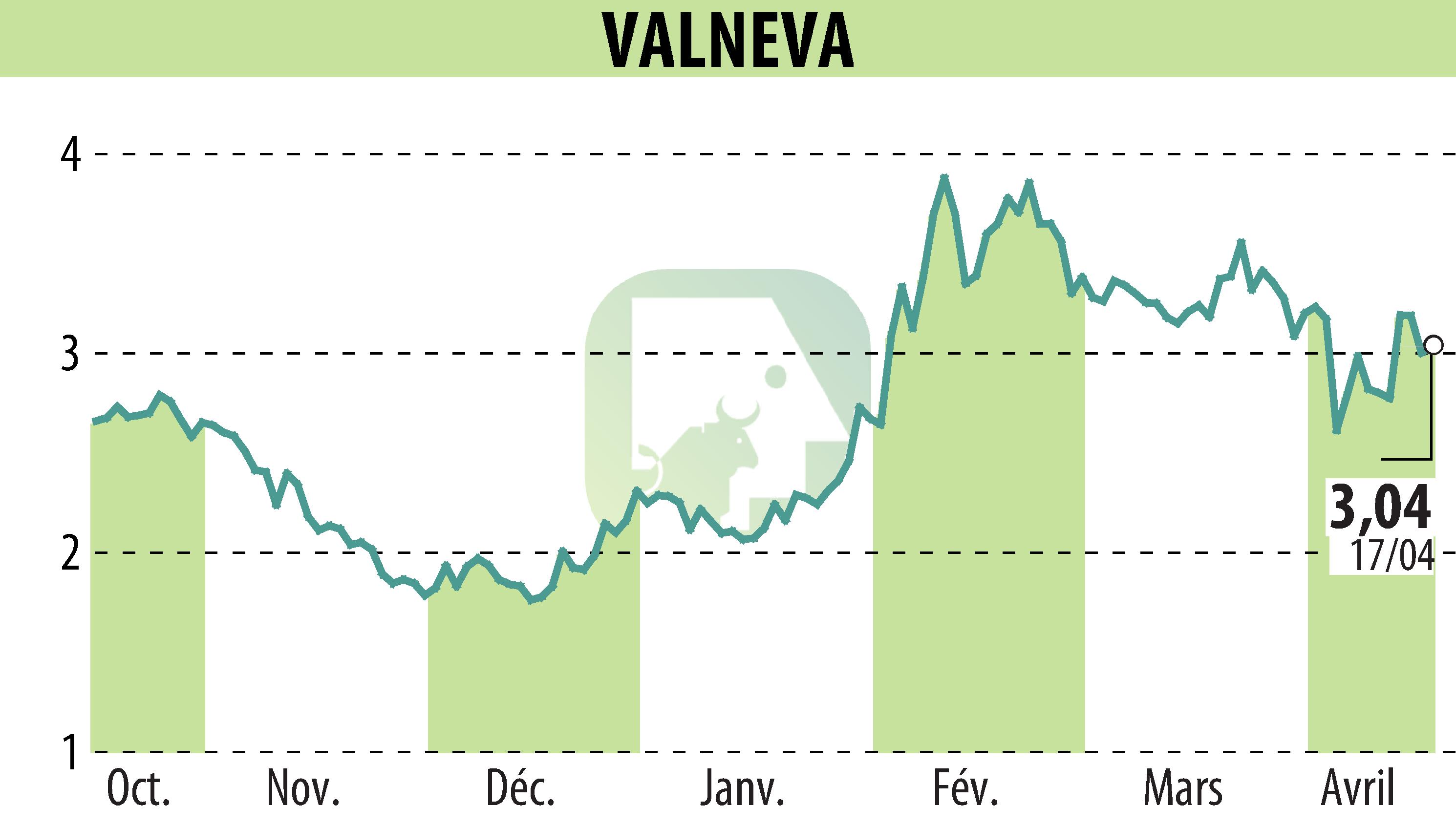
Valneva SE has announced an update regarding its chikungunya vaccine, IXCHIQ®, after a meeting held by the CDC's Advisory Committee on Immunization Practices (ACIP) on April 16. IXCHIQ® remains recommended for adults aged 18 and above traveling to areas experiencing chikungunya outbreaks. It is also considered for those traveling to regions with a higher risk of the virus if the trip is extended.
However, ACIP has advised caution for vaccine use in individuals aged 65+, following reports of serious adverse events in this demographic. This caution has prompted ongoing investigations, even though no direct causal link has been established between the vaccine and these events.
Despite this, IXCHIQ® is still endorsed in pressing situations, such as outbreaks affecting this age group with comorbid conditions. Valneva has currently distributed 80,000 doses globally, with no additional adverse events reported since January 2025.
R. P.
Copyright © 2026 FinanzWire, tous droits de reproduction et de représentation réservés.
Clause de non responsabilité : bien que puisées aux meilleures sources, les informations et analyses diffusées par FinanzWire sont fournies à titre indicatif et ne constituent en aucune manière une incitation à prendre position sur les marchés financiers.
Cliquez ici pour consulter le communiqué de presse ayant servi de base à la rédaction de cette brève
Voir toutes les actualités de VALNEVA
