par MAMMOTOME
Mammotome Launches First In-Room MR Vacuum-Assisted Breast Biopsy System
EQS-News: MAMMOTOME / Key word(s): Product Launch/Miscellaneous
Mammotome Launches First In-Room MR Vacuum-Assisted Breast Biopsy System
18.02.2026 / 08:05 CET/CEST
The issuer is solely responsible for the content of this announcement.
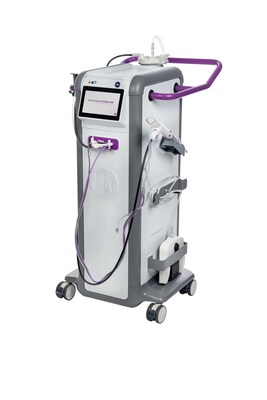
HAMBURG, Germany, Feb. 18, 2026 /PRNewswire/ -- Mammotome, a Danaher company, announces the Mammotome Prima™ MR Dual Vacuum-Assisted Breast Biopsy System, the industry's first system designed to be positioned in the MRI scanner room, at the patient's side during a breast biopsy procedure. This breakthrough innovation enhances clinician coordination, improves communication, and keeps the focus on patient care by bringing the vacuum-assisted breast biopsy device directly into the room.

The Mammotome Prima™ MR system streamlines workflow with an intuitive in-room design1. Clinicians benefit from larger tissue samples2 enabled by stronger vacuum3 and an 8-gauge needle. This is made possible by 75% less tubing compared to other systems4. Less tubing also results in faster setup and cleanup and less biohazard waste. Additional highlights include convenient aperture size and vacuum strength adjustments on a touchscreen interface.
Ultimately, these combined benefits ensure clinicians can focus on delivering safe, accurate, and compassionate care, translating into a better experience for patients. "Our commitment to advancing breast care drives every innovation we bring to market," said Scott Treml, Mammotome Vice President and General Manager. "As the first of its kind in-room solution, the Mammotome Prima™ MR system empowers clinicians with greater efficiency and confidence, ultimately improving the patient experience."
The Mammotome Prima™ MR system has secured CE mark, and first cases are underway in select European countries. The system will be making its debut at the European Society of Radiology (ECR) Congress in March. Mammotome will be expanding access in select global markets throughout 2026. To learn more, please visit the Mammotome website.
1. Compared to the Hologic ATEC® system based on a survey of 16/16 (100%) MRI technologists currently using the Hologic ATEC® system, after using the Mammotome Prima™ system. |
2. Mammotome Prima™ MR 8G probe compared to the Hologic ATEC® 9G yielded 78.6% higher average sample weight based on porcine lab data from 120 tissue samples for each group of devices. |
3. Measured at the aperture, compared to the Hologic ATEC® breast biopsy system based on benchtop testing. |
4. Compared to the Hologic ATEC® standard MR probe tubing and the BD Encor Enspire™ MR probe with tubing extension. |
About Mammotome
At Mammotome, our expertise and compassion for breast care makes us the indispensable partner to physicians, clinicians and patients. Our drive for innovation is rivaled only by our compassion for the people we serve, from the clinicians and surgeons who demand consistently precise solutions, to the patients and families seeking peace of mind. We boast a comprehensive range of products that create better outcomes in breast care and provide physicians and patients with educational resources that guide their journey. Headquartered in Cincinnati, OH, Mammotome has been part of the Danaher Corporation since 2014. The Mammotome brand of products is sold in over 45 different countries throughout the world.

Logo - https://mma.prnewswire.com/media/2905253/Mammotome_Logo.jpg
Photo - https://mma.prnewswire.com/media/2905254/Mammotome_Prima_MR.jpg
Photo - https://mma.prnewswire.com/media/2905255/Mammotome_Prima_MR_System_with_no_tray__accessories_docked_angled_left.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/mammotome-launches-first-in-room-mr-vacuum-assisted-breast-biopsy-system-302689881.html
View original content:https://www.prnewswire.co.uk/news-releases/mammotome-launches-first-in-room-mr-vacuum-assisted-breast-biopsy-system-302689881.html

18.02.2026 CET/CEST Dissemination of a Corporate News, transmitted by EQS News - a service of EQS Group.
The issuer is solely responsible for the content of this announcement.
The EQS Distribution Services include Regulatory Announcements, Financial/Corporate News and Press Releases.
View original content: EQS News
2277818 18.02.2026 CET/CEST

